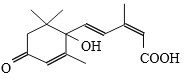
��Ŀ����
����Ŀ����������(Na2FeO4)��һ�����͡���Ч��ˮ����������ˮ��Ӧ�Ļ�ѧ����ʽΪ4Na2FeO4��10H2O===4Fe(OH)3����3O2����8NaOH������Ʊ�Na2FeO4װ��ʾ��ͼ��ͼ��ʾ��
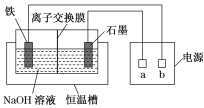
(1)a�ǵ�Դ��________(����������������)�������ʱ��ʯī�缫������Һ�ļ���________(������ǿ��������������������)��
(2)���缫�ķ�ӦʽΪ_________________________________________________��
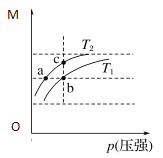
(3)ά��һ���ĵ���ǿ�Ⱥ͵���¶ȣ�NaOH��ʼŨ�ȶ�Na2FeO4Ũ��Ӱ����ͼ(���Һ�����ͬ������½���ʵ��)��
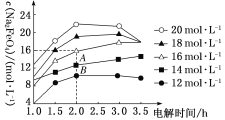
�ٵ��3.0 h�ڣ���NaOH��ʼŨ������Na2FeO4Ũ�ȱ仯������________(������������������������С��)��
�ڵ�NaOH��ʼŨ��Ϊ16 mol��L��1ʱ��1.0��2.0 h������Na2FeO4��������__________mol��L��1��h��1��
(4)�ᴿ�������Na2FeO4�������ؽᾧ�����ˡ�ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ��________(����)��Һ���������
A��Fe(NO3)3�� ��B��NH4Cl�� ��C��CH3COONa
(5)��������������Ҳ�����Ƶ�Na2FeO4��
��֪��2H2(g)��O2(g)===2H2O(l)����H��a kJ��mol��1
NaCl(aq)��H2O(l)===NaClO(aq)��H2(g)����H��b kJ��mol��1
4Na2FeO4(aq)��10H2O(l)===4Fe(OH)3(s)��3O2(g)��8NaOH(aq)����H��c kJ��mol��1
��Ӧ2Fe(OH)3(s)��3NaClO(aq)��4NaOH(aq)===2Na2FeO4(aq)��3NaCl(aq)��5H2O(l)����H��_______kJ��mol��1��
���𰸡��� ��ǿ Fe��8OH����6e��===FeO![]() ��4H2O ���� 8 C ��
��4H2O ���� 8 C ��![]() a��3b��
a��3b��![]() c
c
��������
��ⷨ�Ʊ�Na2FeO4����Ԫ�ػ��ϼ����ߣ���ʧ���ӷ���������Ӧ���������缫��������ʯī�缫Ϊ������
(1) ��ⷨ�Ʊ�Na2FeO4����Ԫ�ػ��ϼ����ߣ��������缫��������ʯī�缫Ϊ������a�ǵ�Դ�ĸ��������ʱ��������Ӧʽ��![]() ��ʯī�缫������Һ�ļ�����ǿ��
��ʯī�缫������Һ�ļ�����ǿ��
(2)���缫��������������ʧ��������Na2FeO4��������ӦʽΪFe��8OH����6e��===FeO![]() ��4H2O��
��4H2O��
(3)�ٸ���ͼʾ�����3.0 h�ڣ���NaOH��ʼŨ������Na2FeO4Ũ������
�ڵ�NaOH��ʼŨ��Ϊ16 mol��L��1ʱ��1.0��2.0 h��Na2FeO4��Ũ�ȱ仯����16 mol��L��1��8 mol��L��1=8 mol��L��1��1.0��2.0 h������Na2FeO4��������8 mol��L��1��1h=8mol��L��1��h��1��
(4)��4Na2FeO4��10H2O===4Fe(OH)3����3O2����8NaOH��Ӧ��֪����������������FeO42-��ˮ��Ӧ�������ü�����Һϴ����ã�Fe(NO3)3��NH4Cl��Һ�����ԣ� CH3COONa��Һ�ʼ��ԣ���ѡC��
(5)��2H2(g)��O2(g)===2H2O(l)����H��a kJ��mol��1
��NaCl(aq)��H2O(l)===NaClO(aq)��H2(g)����H��b kJ��mol��1
��4Na2FeO4(aq)��10H2O(l)===4Fe(OH)3(s)��3O2(g)��8NaOH(aq)����H��c kJ��mol��1
���ݸ�˹���ɣ�-����2 -����3-����![]() ��2Fe(OH)3(s)��3NaClO(aq)��4NaOH(aq)===2Na2FeO4(aq)��3NaCl(aq)��5H2O(l)����H����
��2Fe(OH)3(s)��3NaClO(aq)��4NaOH(aq)===2Na2FeO4(aq)��3NaCl(aq)��5H2O(l)����H����![]() a��3b��
a��3b��![]() c kJ��mol��1��
c kJ��mol��1��

 ֥�鿪���γ�������ϵ�д�
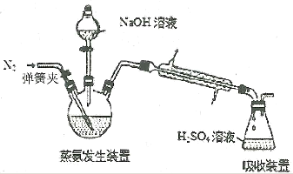
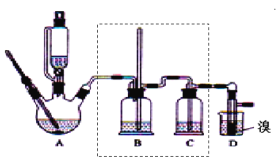
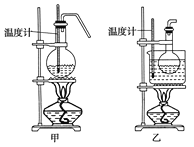
֥�鿪���γ�������ϵ�д�����Ŀ��ʵ�����Ʊ�1��2���������飬�����������Ҵ����Ʊ���ϩ��������ϩ�����������Ʊ�1��2���������飬װ����ͼ��ʾ���й������б������ʾ���ش��������⣺
�Ҵ� | 1��2���������� | ���� | |
״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
�ܶ�/gcm-3 | 0.79 | 2.2 | 0.71 |
�е�/�� | 78.5 | 132 | 34.6 |
�۵�/�� | -130 | 9 | -116 |
��1���ڴ��Ʊ�ʵ���У�Ҫ������Ѹ�ٵذѷ�Ӧ�¶���ߵ�170�����ң�������ҪĿ����__��
a.������Ӧ b.�ӿ췴Ӧ�ٶ�
c.��ֹ�Ҵ��ӷ� d.���ٸ�������������
��2����װ��A�г���Ũ������Ҵ��⣬��Ӧ����__����Ŀ����__��װ��A�����ɸ��������ѵĻ�ѧ��Ӧ����ʽΪ__��
��3��ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2��Ϊ����֤SO2�Ĵ��ڲ���ȥSO2�Ժ�����Ӧ�ĸ��ţ�ijͬѧ��A��D֮�������B��C����װ�ã�����B��C�пɷֱ�ʢ��___��
a.����KMnO4��ˮ b.Ʒ���NaOH��Һ
c.����KMnO4��NaOH��Һ d.Ʒ�������KMnO4
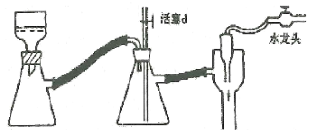
��4���ס�����װ�þ�������ʵ��������ˮ�Ҵ���ȡ��ϩ����ͼ���ø���ԡ����(���ͷе�290�棬�۵�18.17��)���������¶ȴﵽ��Ӧ�¶�ʱ����ʢ����ˮ�Ҵ���Ũ������Һ����ƿ��������У��ܿ�ﵽ��Ӧ�¶ȡ��ס�����װ����Ƚϣ���װ������Щ�ŵ�__��д����ʵ��������ˮ�Ҵ���ȡ��ϩ�Ļ�ѧ����ʽ___��
��5����1��2����������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ú���Ӧ��__�㣻�����������������������ѡ�����__�ķ�����ȥ��