��Ŀ����
����Ŀ�������и�ͼ��ʾ����ѧ��ѧ�г����ڻ���������ᴿ��װ�ã�
A. 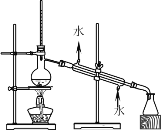 B.
B. 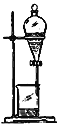
C.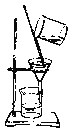 D.
D. 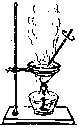
�����װ��ͼ�ش��������⣺
��Aͼ������ʢ��Һ�����������������Ϊ��__________________
��ѡ����ʵ�ʵ��װ�÷������»��������װ�õ���ĸ����
�ٴӵ�ˮ�з����I2 �� ___________
�ڽ��оƾ���ˮ�ķ��룺 ___________
�۶������̺�������Һ�� ___________
���ҹ�������ʷ�ƾõľ��Ļ�����ش��������⣺
��Ҫ��߾ƾ��Ķ������ɲ��õķ�����________________���������������ͬ��
����ҩ�����������������Ƴ�ҩ�ƣ�����Ϊ���õķ�����_____________��
���𰸡�������ƿBAC������ȡ
��������
��1��AͼΪ����װ�ã�����ʢ��Һ�����������Ϊ������ƿ��
��2�����ݻ�����и��������ʵIJ���ѡ����뷽����װ��ͼ��
��3�����ݸ��������ʵIJ���ѡ����뷽����
��1��AͼΪ����װ�ã�����ʢ��Һ�����������������Ϊ������ƿ��
��2��AͼΪ����װ�ã�BͼΪ��Һװ����CͼΪ����װ�ã�DͼΪ����װ����
����������CCl4�������л��ܼ����ӵ�ˮ�з����I2��Ӧ���ˮ�м�����ȡ������CCl4�����ȣ���ȡ���⣬Ȼ���Һ����ѡB��
���ƾ���ˮ�Ƿе㲻ͬ������ܵ�Һ���������ƾ���ˮӦ����������ѡA��
��MnO2Ϊ������ˮ�Ĺ��壬����MnO2��������ҺӦ�ù��˷�����ѡC��
��3����Ҫ��߾ƾ��Ķ����������پƾ���ˮ�Ļ������H2O���ƾ���ˮ�Ƿе㲻ͬ������ܵ�Һ���������ɲ��õķ���������
����ҩ�ж��ֳɷ������ھƾ������Ҵ������������ƽ�����ҩ�ɽ���ҩ�ж��ֳɷֽ�ȡ�����У���ҩ�����������������Ƴ�ҩ�����õķ�������ȡ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��������������Ӱ�����ǵ�����ͽ�����������Ҫ��Ⱦ��Ϊ�����������PM2.5������Ҫ��ԴΪȼú��������β���ȡ���˸�����Դ�ṹ���������ŵȴ�ʩ����Ч����PM2.5��SO2��NOx����Ⱦ��
��ش���������:
(1)��һ������ijPM2.5 ��Ʒ������ˮ�ܽ��Ƴɴ�������(����OH-)�������²�ø���������ɼ���Ũ�����±�:���ݱ��������жϸ�������pH=________��
���� | K+ | Na+ | NH4+ | SO42- | NO3- | CI- |
Ũ��mol/L | 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
(2)��֪����������NO�����ɷ�ӦΪ:N2(g)+ O2(g) ![]() 2NO(g) ��H>0���£������ܱ������У�����˵���У���˵���÷�Ӧ�ﵽ��ѧƽ��״̬����______________��
2NO(g) ��H>0���£������ܱ������У�����˵���У���˵���÷�Ӧ�ﵽ��ѧƽ��״̬����______________��
A.���������ܶȲ��ٱ仯 B.��������ѹǿ���ٱ仯
C.������ת���ʲ��ٱ仯 D.N2��O2��NO�����ʵ���֮��Ϊ1: 1: 2
(3)Ϊ����SO2 ���ŷţ�����ȡ�Ĵ�ʩ��:
�ٽ�úת��Ϊ�������ȼ�ϡ�
��֪:H2(g)+1/2O2(g)==H2O(g) ��H= -241.8kJ/mol
C(s)+1/2O2(g)==CO(g) ��H=-110.5kJ/mol
д����̿��ˮ������Ӧ���Ȼ�ѧ����ʽ:__________________________��
��ϴ�Ӻ�SO2�����������п���Ϊϴ�Ӻ�SO2������ϴ�Ӽ���________��
A.Ũ��ˮ B.̼�����Ʊ�����Һ
C.FeCl2������Һ D.����CaCl2������Һ
(4)����ʹ���Ҵ����Ͳ����ܼ���NOx���ŷţ���ʹNOx����Ч������Ϊ�����������Ҫ���⡣ij�о���С����ʵ������Ag-ZSM-5Ϊ���������NOת��ΪN2��ת�������¶ȱ仯�����ͼ��ʾ������ʹ��CO���¶ȳ���775K������NO�ķֽ��ʽ��ͣ�����ܵ�ԭ��Ϊ____________����n(NO)/n(CO)=1�������£�Ϊ���õij�ȥNOx���ʣ�Ӧ���Ƶ�����¶���______K���ҡ�

(5)�����ŷŵĵ������úȼ�ղ����Ķ��������ǵ������������ġ�������ס�������̿�ɴ���������Ⱦ��NO����5L�ܱ������м���NO �ͻ���̿(����������)��һ����������������E��F�����¶ȷֱ���T1���T2��ʱ����ø�����ƽ��ʱ���ʵ���(n/mol)���±�:

��д��NO�����̿��Ӧ�Ļ�ѧ����ʽ____________________________��
����T12����÷�Ӧ�ġ�H_____________0(�>������<����=��)��
��������ӦT1��ʱ�ﵽ��ѧƽ�����ͨ��0.lmol NO���壬��ﵽ�»�ѧƽ��ʱNO��ת����Ϊ___________________��