��Ŀ����
����Ŀ������A��B��C��������A��B�����е�������ԭ�ӱ�C��һ��ȡ��������������ʧȥһ����ԭ�Ӻ�ʣ��IJ��֣�ȡ���IJ��
��֪���� A����ʹ���CCl4��Һ��ɫ����һ�ȴ���ֻ��һ�֡�
�� һ������B��ȫȼ�գ�����������ͨ��ʢ��CaCl2�ͼ�ʯ�ҵĸ���ܣ�����������طֱ�Ϊ3.6g��17.6g����26g/mol��M��B����78g/mol��
�� CΪ����������ͨ�������Ϊ��̬����ͬ���칹�岻����2�֣����������3�֡�
�ش��������⣺
��1��B�����ʽ��_______��д��B��һ����״ͬ���칹��Ľṹ��ʽ_________��Ҫ�������ֹ����ţ� ��
��2��C�ķ���ʽΪ_______����C������̼ͬԭ����������̼ԭ����Ҳ��ͬ�ĵ�ϩ���������� ��
��3��A�Ľṹ��ʽ______________��
���𰸡���1��CH CH2=CHC��CH
��2��C4H10 2-��-1-��ϩ
��3��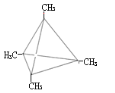
��������
���������B��ȫȼ�յIJ�����n��CO2����n��H2O����(17.6g��44g/mol):(3.6g��18g/mol)��2��1������B����ɿ��Ա�ʾΪ��CH��n������26��M��A����78������26��13n��78�����2��n��6��B��̼ԭ�Ӹ�������Ϊ3��4��5����������ԭ�Ӹ���Ϊż����B�ķ���ʽֻ��ΪC4H4��C�DZ���������ͨ������³���̬��̼ԭ����ĿС��5����������⣩����ͬ���칹�壬̼ԭ����Ŀ����3��������������֣�����C�Ľṹ��ʽΪCH��CH3��3���������⣬C���������Ϊ-CH3��A��������Br2��CCl4��Һ����ɫ��˵�������в��������ͼ�����һ�ȴ���ֻ��һ�֣�˵��������Hԭ��Ϊ��ЧHԭ�ӣ��л���A����Ϊ����B�����е�������ԭ�ӱ���C�������������ȡ�����õ��ģ�B�ķ���ʽΪC4H4����AΪC4(CH3)4 ��
��1����������������֪��B����ʽΪC4H4�����ʽ��CH��B��һ����״ͬ���칹��Ľṹ��ʽ������ԭ����ͬһƽ���ڣ�����̼ԭ�Ӳ�����ͬһֱ���ϣ���������к���1��̼̼˫����1��̼̼��������̼̼������1��C����ṹ��ʽΪCH2=CHC��CH ��
��2����������������C�ķ���ʽΪC4H10���ṹ��ʽΪCH(CH3)3����C������̼ͬԭ����������̼ԭ����Ҳ��ͬ�ĵ�ϩ��Ϊ��CH3��2C��CH2��������Ϊ��2-��-1-��ϩ��
��3�����������ƶϣ�AΪC4(CH3)4 ����ṹ��ʽΪ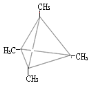 ��
��

����Ŀ����ϸ��������ʵ��:
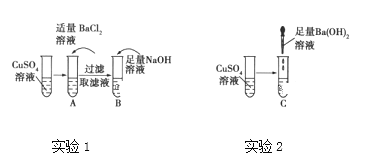
A��B��C�Թ��е����������ʾ:
�Թ� | A | B | C |
���� | ������ɫ����, ��Һ��Ϊ��ɫ | ������ɫ����, ��Һ��Ϊ��ɫ | ������ɫ����, ��Һ��Ϊ��ɫ |
д��A��B��C�Թ�����������Ӧ�����ӷ���ʽ��
��1��A��______________________________________________��
��2��B��______________________________________________��
��3��C��______________________________________________��
����Ŀ��ijʵ��С����H2O2�ֽ�Ϊ�����о�Ũ�ȡ���������Һ����ԶԷ�Ӧ���ʵ�Ӱ�졣�ڳ����°������·������ʵ�顣
ʵ���� | ��Ӧ�� | ���� |
�� | 10 mL 2%H2O2��Һ | �� |
�� | 10 mL 5% H2O2��Һ | �� |
�� | 10 mL 5% H2O2��Һ | 1 mL 0.1 mol��L��1FeCl3��Һ |
�� | 10 mL 5% H2O2��Һ������HCl��Һ | 1 mL 0.1 mol��L��1FeCl3��Һ |
�� | 10 mL 5% H2O2��Һ������NaOH��Һ | 1 mL 0.1 mol��L��1FeCl3��Һ |
(1)ʵ��ٺ͢ڵ�Ŀ����______________________��
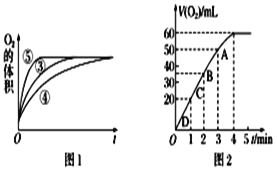
(2)ʵ��ۢܢ��У�������������������ʱ��仯�Ĺ�ϵ��ͼ1������ͼ1�ܹ��ó���ʵ������ǣ���____________������_____________��
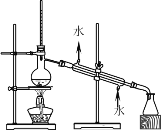
(3)����0.1 g MnO2��ĩ��50 mL H2O2��Һ�У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ2��ʾ����Ӧ�����仯��ԭ����________________��