��Ŀ����
16����ú����ȼú�糧�ķ�������Ҫ�ɷ�ΪSiO2��Al2O3��Fe2O3��C�ȣ�ʵ����ģ�ҵ�ӷ�ú����ȡ����Al2O3����������ͼ��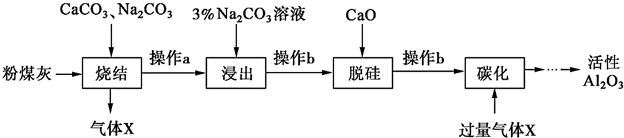
��֪�ս���̵IJ�����Ҫ�ǣ�NaAlO2��Ca2SiO4��NaFeO2��Na2SiO3�ȣ�
��1��д���ս��������Ԫ��ת���Ļ�ѧ����ʽAl2O3+NaCO3$\frac{\underline{\;����\;}}{\;}$2Na AlO2+CO2����
��2������aΪ��ȴ����ĥ��������ĥ��Ŀ��������ս��������ʣ�
��3�����������У�NaFeO2����ȫˮ�⣬ˮ�ⷴӦ�����ӷ���ʽΪFeO2-+2H2O?Fe ��OH��3��+OH-��
��4������b�������ǹ��ˣ����õIJ���������©�������������ձ���
��5����̼����ʱ�����������������������Ļ�ѧʽΪAl��OH��3��
���� ��ú����ȼú�糧�ķ�������Ҫ�ɷ�ΪSiO2��Al2O3��Fe2O3��C�ȣ�����CaCO3��Na2CO3�����սᣬ����Al2O3+Na2CO3$\frac{\underline{\;����\;}}{\;}$2NaAlO2+CO2����Na2CO3+SiO2$\frac{\underline{\;����\;}}{\;}$Na2SiO3+CO2����2CaCO3+SiO2$\frac{\underline{\;����\;}}{\;}$Ca2SiO4+2CO2����Fe2O3+Na2CO3$\frac{\underline{\;����\;}}{\;}$2NaFeO2+CO2����C+O2$\frac{\underline{\;����\;}}{\;}$CO2����������XΪCO2������aΪ��ȴ����ĥ������̼������Һ������AlO2-+2H2O?Al��OH��3+OH-��̼������Һ�ʼ��ԣ�����ƫ��������ӵ�ˮ�⣬NaFeO2����ȫˮ�⣬FeO2-+2H2O=Fe��OH��3��+OH-������b���˳�Fe��OH��3��Ca2SiO4�������������ѹ裬�����ƺ�ˮ��Ӧ�����������ƣ��������ƺ�������ӷ�Ӧ���ɹ���Ƴ���������b���˳�����Ƴ�������ҺΪNaAlO2��ͨ�������̼��̼������ǿ���������������Է�����NaAlO2+CO2+2H2O=NaHCO3+Al��OH��3�������˳���������������������������������������ˮ������������õ������������ݴ˷�����
��� �⣺��ú����ȼú�糧�ķ�������Ҫ�ɷ�ΪSiO2��Al2O3��Fe2O3��C�ȣ�����CaCO3��Na2CO3�����սᣬ����Al2O3+Na2CO3$\frac{\underline{\;����\;}}{\;}$2NaAlO2+CO2����Na2CO3+SiO2$\frac{\underline{\;����\;}}{\;}$Na2SiO3+CO2����2CaCO3+SiO2$\frac{\underline{\;����\;}}{\;}$Ca2SiO4+2CO2����Fe2O3+Na2CO3$\frac{\underline{\;����\;}}{\;}$2NaFeO2+CO2����C+O2$\frac{\underline{\;����\;}}{\;}$CO2����������XΪCO2������aΪ��ȴ����ĥ������̼������Һ������AlO2-+2H2O?Al��OH��3+OH-��̼������Һ�ʼ��ԣ�����ƫ��������ӵ�ˮ�⣬NaFeO2����ȫˮ�⣬FeO2-+2H2O=Fe��OH��3��+OH-������b���˳�Fe��OH��3��Ca2SiO4�������������ѹ裬�����ƺ�ˮ��Ӧ�����������ƣ��������ƺ�������ӷ�Ӧ���ɹ���Ƴ���������b���˳�����Ƴ�������ҺΪNaAlO2��ͨ�������̼��̼������ǿ���������������Է�����NaAlO2+CO2+2H2O=NaHCO3+Al��OH��3�������˳���������������������������������������ˮ������������õ�����������
��1�����սᡰ��������������̼���Ʒ�Ӧ��Al2O3+Na2CO3$\frac{\underline{\;����\;}}{\;}$2NaAlO2+CO2�����ʴ�Ϊ��Al2O3+Na2CO3$\frac{\underline{\;����\;}}{\;}$2NaAlO2+CO2����
��2�����սᡱ���̵IJ�����Ҫ�ǣ�NaAlO2��Ca2SiO4��NaFeO2��Na2SiO3�ȣ�����a����һ��Ϊ����������aΪ��ȴ����ĥ��������ĥ��Ŀ��������ս��������ʣ�
�ʴ�Ϊ������ս��������ʣ�
��3��̼������Һ�ʼ��ԣ�����ƫ��������ӵ�ˮ�⣬NaFeO2����ȫˮ�⣬FeO2-+2H2O=Fe��OH��3��+OH-��
�ʴ�Ϊ��FeO2-+2H2O=Fe��OH��3��+OH-��
��4������Һ�������β���b�������������bΪ���ˣ�����ʱ��Ҫ������������©�����̶�����������̨�������õIJ��������н���Һ���ձ������Ի����õIJ���������©������������
�ʴ�Ϊ�����ˣ�©������������
��5������b���˳�����Ƴ�������ҺΪNaAlO2��ͨ�������̼��̼������ǿ���������������Է�����NaAlO2+CO2+2H2O=NaHCO3+Al��OH��3�������ԡ�̼����ʱ���ɳ�������������
�ʴ�Ϊ������������Al��OH��3��
���� ���⿼�������ʵķ��롢�ᴿ�ķ�����Ӧ�ã�����ѧ���Թ������̵����⡢Ԫ�ػ��������ʵȣ���ȷ�����ʵ������ǽⱾ��Ĺؼ����ѶȽϴ�

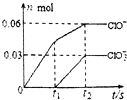 ��һ������Cl2ͨ��һ��Ũ�ȵĿ��Լ���Һ�У�����ǡ����ȫ��Ӧ����֪��Ӧ���̷��ȣ����������������ֺ���Ԫ�ص����ӣ�����ClO-��ClO${\;}_{3}^{-}$�������ӵ����ʵ�����n���뷴Ӧʱ�䣨t���ı仯ʾ��ͼ��ͼ��ʾ������˵����ȷ���ǣ�������
��һ������Cl2ͨ��һ��Ũ�ȵĿ��Լ���Һ�У�����ǡ����ȫ��Ӧ����֪��Ӧ���̷��ȣ����������������ֺ���Ԫ�ص����ӣ�����ClO-��ClO${\;}_{3}^{-}$�������ӵ����ʵ�����n���뷴Ӧʱ�䣨t���ı仯ʾ��ͼ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ���Լ���Һ��KOH��������4.94g | |
| B�� | ��Ӧ��ת�Ƶ��ӵ����ʵ�����0.21mol | |
| C�� | Cl-�����ʵ���Ϊ0.09mol | |
| D�� | ClO${\;}_{3}^{-}$������������KOH��������� |
| A�� | �����ͳ�����O3������Ԫ�ص�ͬ�������� | |
| B�� | ����ͼ��������ͬ���칹�� | |
| C�� | 12C��13C��Ϊͬλ�� | |
| D�� | ��֬�ᣨC15H31COOH�������ᣨC17H33COOH����ͬϵ�� |
| A�� | ��Al��Cu��ϡH2SO4���ԭ��أ��为����ӦʽΪ��Al-3e-=Al3+ | |
| B�� | ��Mg��Al��NaOH��Һ���ԭ��أ��为����ӦʽΪ��Al-3e-+3OH��=Al��OH��3 | |
| C�� | ��Fe��Cu��FeCl3��Һ���ԭ��أ��为����ӦʽΪ��Fe-2e-=Fe2+ | |
| D�� | ��Fe��Cu��Ũ������ɵ�ԭ��أ���ʼʱ�为����ӦʽΪ��Cu-2e-=Cu2+ |
| A�� | $\frac{3}{8}$ mol | B�� | $\frac{8}{3}$ mol | C�� | $\frac{2}{3}$ mol | D�� | $\frac{3}{2}$ mol |
| A�� | �ԷϾɵ�ؽ��л��մ��� | |
| B�� | ��ֹ���������ۡ�ʹ�ó������Ϲ���� | |
| C�� | �Ծ���ϩ��������������������㵹�뺣 | |
| D�� | ʹ��������̫���ܡ����ܵ���Դ�����ͳ��ú̿ |
| A�� | ���ؽᾧ�ķ������뱽������Ȼ��ƵĻ���� | |
| B�� | �ñ�����ˮ��Fe���¿��Ƶ��屽 | |
| C�� | ���Ҵ����������pH=1��H2SO4��Һ��ϼ��ȿ��Ʊ��������� | |
| D�� | ����ʯ��ˮ��Ӧ�������ֱ��ͨ�뵽��ˮ�У�������֤��Ȳ��ʹ��ˮ��ɫ������ |

 ��
��