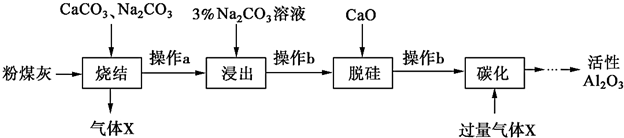
��Ŀ����
7��ij�о���ѧϰС����ȡ�κ���±��Ũ��Һ������K+��Mg2+��Br-��SO${\;}_{4}^{2-}$��Cl-�ȣ�����ȡ�ϴ������Ȼ��ؾ��弰Һ�壨Br2���������������ͼ���̣�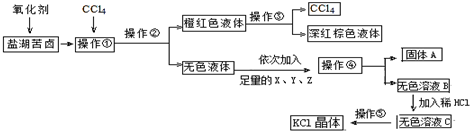
������������̣��ش�������⣺
��1������������ȡ���������Ƿ�Һ���������������ᾧ������������ƣ�
��2����������Ҫ����Ҫ�����������ձ��⣬����Ҫ��Һ©����
��3������������X��Y��Z��Ϊ�˳�ȥ��Һ�е����ʣ�����������BaCl2��Һ��KOH��Һ��K2CO3��Һ��
��4��������ɫ��ҺB���Ƿ���SO${\;}_{4}^{2-}$�ķ�����ȡ������ҺB���Թ��У����Թ��ڵμ�BaCl2��Һ��ϡ���ᣬ���ް�ɫ������������SO42-�����а�ɫ������������SO42-��
���� �ʼ�ӵ���������Br-��������Br2���ɼ���CCl4��֪��������ȡ����ȡBr2�����Բ���������ȡ�������ڽ�CCl4���ˮ��ֿ��Ĺ��̽�����Һ�������������ۣ������CCl4��Һ�з���õ�CCl4��Һ�壻
�����ڵõ�����ɫ��Һ����������SO42-�����õ�����ɫ��ҺC���������ᾧ�õ�KCl���壮
��� �⣺��1�������̿�֪���ʼ�ӵ���������Br-��������Br2���ɼ���CCl4��֪��������ȡ����ȡBr2�����Բ���������ȡ����CCl4���ˮ��ֿ��Ĺ��̽�����Һ���������Ϊ��Һ�������ݽ��õ�����ɫ��ҺC���������ᾧ�õ�KCl���壻
�ʴ�Ϊ����ȡ����Һ�� �����ᾧ��
��2��������Ϊ��Һ����Ҫ�õ���Һ©����
�ʴ�Ϊ����Һ©����
��3��BaCl2��Һ��ȥSO42-��KOH��Һ��ȥMg2+���ټ���K2CO3��Һ����ȥ������BaCl2��
�ʴ�Ϊ��K2CO3��
��4��������ɫ��ҺB���Ƿ���SO42-�����ǣ�ȡ������ҺB���Թ��У����Թ��ڵμ�BaCl2��Һ��ϡ���ᣬ���ް�ɫ������������SO42-�����а�ɫ������������SO42-��
�ʴ�Ϊ��ȡ������ҺB���Թ��У����Թ��ڵμ�BaCl2��Һ��ϡ���ᣬ���ް�ɫ������������SO42-�����а�ɫ������������SO42-��
���� �������弰�仯�������������ᴿ�����������еķ��뷽��Ϊ���Ĺؼ���ע�ⳣ�����ʵ����ʼ������ķ��뷽�����ɽ����Ŀ�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ��4molHCl��Ũ����������MnO2��ַ�Ӧ��ת��2NA������ | |
| B�� | 500�桢30MPa�£���0.2mol N2��0.6molH2�����ܱյ������г�ַ�Ӧ����NH3��g��������7.72kJ�����Ȼ�ѧ����ʽΪ�� N2��g��+3H2��g�� $?_{500�桢30MPa}^{����}$ 2NH3��g����H=-38.6kJ•mol-1 | |
| C�� | ���ڿ��淴ӦN2��g��+3H2��g��$?_{���¸�ѹ}^{����}$2NH3��g������H��O�������¶ȣ���ʹ��Ӧ��������Ӧ�����ƶ� | |
| D�� | Ԫ��ԭ�ӵ������������Ķ�������ǽ����Ե�ǿ���ޱ�Ȼ��ϵ |
2Na2S��aq��+Na2CO3��aq��+4SO2��aq���T3Na2S2O3��aq��+CO2��g����H��0
ʵ��С����ʵ��������Ʊ�Na2S2O3.5SH2O�������£�
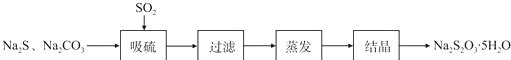
��1������װ����ͼ��ʾ��

��װ��B�������Ǽ���װ��A��SO2������Ч�ʣ�װ��C�����������ն�������ֹ��Ⱦ����
��Ϊ�����SO2�������ʣ��ڲ��ı�A����ҺŨ�ȡ�����������£����˼�ʱ���跴Ӧ����ɲ��ú����Ĵ�ʩ��
����SO2�ĽӴ����������SO2�����١��ʵ������¶ȵȣ�д��������
�ۼ��豾ʵ�����õ�Na2CO3������NaCl��NaOH�����ʵ�鷽�����м���
��֪������ʱBaCO3������Һ��pH=9.6����ѡ�Լ���������ϡ���ᡢAgNO3��Һ��BaCl2��Һ����̪��Һ������ˮ��pH�ơ��ձ����Թܡ��ι�
| ��� | ʵ����� | Ԥ������ | ���� |
| �� | ȡ������Ʒ���Թ��У�������������ˮ��������ܽ� �μ�����ϡ���ᣬ�ٵμ�����AgNO3��Һ���� | �а�ɫ�������� | ��Ʒ��NaCl |
| �� | ȡ������Ʒ���Թ��У�������������ˮ��������ܽ� �������BaCl2��Һ�����裬���ã���pH�Ʋⶨ�ϲ���ҺpH | �а�ɫ�������ɣ��ϲ���ҺpH����9.6 | ��Ʒ��NaOH |
��֪��IO3+5I+6H+�T3I2+3H2O 2S2O32-+I2�TS4O62-+2I-��
| A�� | MgCl2 | B�� | CO2 | C�� | KOH | D�� | ���ʯ |
| A�� | 5730��3�� | B�� | 5730��4�� | C�� | 5730��6�� | D�� | 5730��8�� |
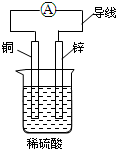
| A�� | пƬΪ������ͭƬΪ���� | |
| B�� | ��װ���ܽ�����ת��Ϊ��ѧ�� | |
| C�� | ͭƬ�����ķ�ӦΪCu-2e-�TCu2+ | |
| D�� | ������������пƬͨ����������ͭƬ |
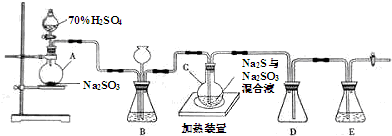

 ���÷�Ӧ����ȡ����Ӧ���л������������������
���÷�Ӧ����ȡ����Ӧ���л������������������