��Ŀ����
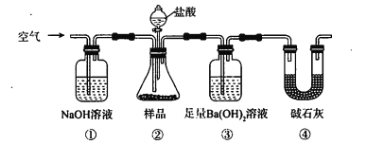
����Ŀ������ʯ����ȡ�طʺ�������������Ҫԭ�ϣ�����ʯ����ɺ��������ƣ�����������������������������ʡ�����ʵ�鲽������ͼ��ʾ��
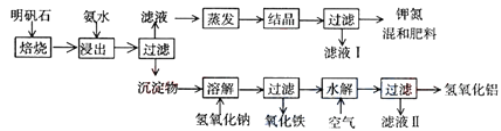
��������ͼʾ�����������գ�
��1������ʯ���պ���ϡ��ˮ����������500mLϡ��ˮ��ÿ������39.20g������ҪȡŨ��ˮ��ÿ������251.28g����__________mL���ù��Ϊ_______mL��Ͳ��ȡ��
��2��д���ܽⲽ���з�Ӧ�õ�������Ϊ_________________��д��ѧʽ��
��3��Ϊ�ⶨ��Ϸ���K2SO4��(NH4)2SO4�мصĺ��������������в��裺
�ٳ�ȡ�ص�������������ˮ����������______��Һ��������ɫ������
��___________��__________��_________(������дʵ���������)��
����ȴ�����ء�
��4��������Ϊmg�����������ʵ���Ϊnmol����������K2SO4�����ʵ���Ϊ��___________mol���ú���m��n�Ĵ���ʽ��ʾ����
���𰸡�78 100 NaAlO2 BaCl2��Ba(NO3)2 ���� ϴ�� ���� ![]()
��������
��1������500mLϡ��ˮ��ÿ������39.20g������ҪȡŨ��ˮ��ÿ������251.28g�������������ϡ��ǰ��������Ƚ��м��㣻���ͽ�ԭ�ó���ѡ��Ͳ�Ĺ��
��2���ܽⲽ���У�����������������������Һ�ķ�Ӧ��
��3��Ϊ�ⶨ��Ϸ���K2SO4��(NH4)2SO4�мصĺ������ɰ������в��裺
�ٳ�ȡ�ص�������������ˮ�����������Լ�����SO42-������ɫ������
�ڳ�������ˡ�ϴ�ӡ�����Զ�Ӧ����������
����ȴ�����ء�
��4����������K2SO4�����ʵ���ʱ���ɼ���K2SO4�����ʵ���Ϊx����(NH4)2SO4Ϊn-x�����������غ㽨����ϵʽ���ɴ����x��
��1������ϡ��ǰ��������ȣ��ɽ������µ�����ϵʽ��
251.28g/L��V(Ũ)= 39.20g/L��0.5L��V(Ũ)=0.078L=78mL���ù��Ϊ100mL��Ͳ��ȡ����Ϊ��78��100��
��2���ܽⲽ���У�������������������Һ�ķ�ӦΪAl2O3+2NaOH==2NaAlO2+H2O����Ӧ�õ�������ΪNaAlO2����Ϊ��NaAlO2��
��3�ٳ�ȡ�ص�������������ˮ����������BaCl2��Ba(NO3)2��Һ��������ɫ��������Ϊ��BaCl2��Ba(NO3)2��
�ڹ��ˡ�ϴ�ӡ������Ϊ�����ˣ�ϴ�ӣ������Ϊ�����ˣ�ϴ�ӣ����
����ȴ�����ء�
��4����K2SO4�����ʵ���Ϊx����(NH4)2SO4�����ʵ���Ϊn-x��
��174x+132(n-x)=m���Ӷ����x=![]() mol����Ϊ��
mol������![]() ��
��

����Ŀ����1��ij��ѧ�о���ѧϰС��ģ�ҵ�ϳɰ��ķ�Ӧ�����ݻ��̶�Ϊ2L���ܱ������ڳ���1molN2��3mol H2��������ʴ���(������Ժ��Բ���)����һ���¶�ѹǿ�¿�ʼ��Ӧ������ѹ���Ƽ��������ѹǿ�ı仯���£�
��Ӧʱ�� /min | 0 | 5 | 10 | 15 | 20 | 25 | 30 |
ѹǿ /MPa | 16.80 | 14.78 | 13.86 | 13.27 | 12.85 | 12.60 | 12.60 |
��ӷ�Ӧ��ʼ��25 minʱ����N2��ʾ��ƽ����Ӧ���ʣ�____��
��2����ҵ�ϳɰ��ķ�Ӧ����ʽΪ��N2(g)+3H2(g)![]() 2NH3(g) ��H����ͼ1�Ǻϳɰ���Ӧ�������뷴Ӧ�������ͼ��δʹ�ô�������ͼ2�Ǻϳɰ���Ӧ��2L�����С���ͬͶ������¡���������������ʱ��ijһ��Ӧ�����ĸı�Է�Ӧ��Ӱ��ͼ��
2NH3(g) ��H����ͼ1�Ǻϳɰ���Ӧ�������뷴Ӧ�������ͼ��δʹ�ô�������ͼ2�Ǻϳɰ���Ӧ��2L�����С���ͬͶ������¡���������������ʱ��ijһ��Ӧ�����ĸı�Է�Ӧ��Ӱ��ͼ��

����˵����ȷ������_____��
A.��H=-92.4kJ/mol
B.ʹ�ô�����ʹE1����ֵ����
C.Ϊ�����ת���ʣ���ҵ�����з�Ӧ���¶�Խ��Խ��
D.ͼ���Dz�ͬѹǿ�·�Ӧ��ϵ�а������ʵ����뷴Ӧʱ���ϵͼ����PA<PB
E.ͼ���Dz�ͬ�¶��·�Ӧ��ϵ�а������ʵ����뷴Ӧʱ���ϵͼ����TA>TB
F.�÷�Ӧ��ƽ�ⳣ��KA<KB
G.������A�����£���Ӧ�ӿ�ʼ��ƽ�⣬����N2��ƽ������Ϊn1/(4t1) mol/(L.min)
��3��һ���¶��£���һ���ݻ�Ϊ2L���ܱ�������ͨ��2molN2��7molH2���ﵽƽ��ʱ��������ڵ�ѹǿΪ��ʼʱ��7/9��������¶��µ�ƽ�ⳣ��Ϊ___����ͬһ�¶ȣ�ͬһ�����У�����ʼ���ʸ�ΪamolN2��bmolH2��cmolNH3��a��b��c����Ϊ�㣩��ʹƽ�������и����ʵ�������ԭƽ����ͬ����a��b��c����Ĺ�ϵΪ____���ú�a��b��c�ı���ʽ��ʾ��������ʹ��Ӧ����ʼʱ���淴Ӧ������У�c��ȡֵ��Χ��____��