��Ŀ����
����Ŀ���о���ѧϰС���ͬѧ��Ϊ�ⶨij��þ3��~5������þ�Ͻ𣨲�������Ԫ�أ���þ����������������������ֲ�ͬʵ�鷽������̽������д���пհס�
��ʵ�鷽��������þ�Ͻ�������ϡ������Һ��Ӧ���ⶨ����������ͨ��״����Լ20����1.01![]() 105Pa���������
105Pa���������
���������ۣ�
��1��ͬѧ����ѡ������ʵ��װ�����ʵ�飺
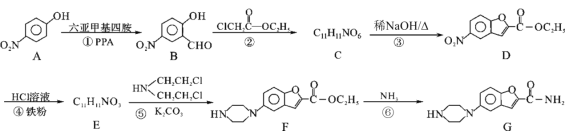
�������װ����Ҫ�����������������ǵ�������˳���ǣ�A�ӣ� ���� ���ӣ� ��____________
��ʵ�鿪ʼʱ���ȴ�Һ©���ϿڵIJ���������������������һ�����ϡ����Ҳ����˳��������ƿ���²���ܵ�ԭ����__________________________________________��
��ʵ�����ʱ���ڶ�ȡ����ʵ�����������������ʱ������Ϊ��������___________ ��
A����ʵ��װ����ȴ���ٶ���
B�������ƶ���ͲF��ʹ����Һ������ƿ��Һ����ƽ
C�������ƶ���ͲG��ʹ����Һ������ƿ��Һ����ƽ
D�������밼Һ�����͵�ˮƽ����ȡ��Ͳ��ˮ�����
��2����ϸ����ʵ��װ�ú�ͬѧ�Ǿ�������Ϊ�������������ϴ���ϡ���������ƿ�У���ʹ������������Ҳ�Ὣƿ�ڿ����ų���ʹ�����������ƫ��ʵ�����ʱ�����ӹ��ƿ����Ͳ�ĵ�����������ˮ���ڣ�ʹ�����������ƫС�����������������ͼ��ʾ��ʵ��װ�á�
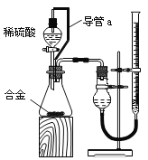
��װ���е���a��������_______________________________����Һ©������ʱϡ������˳�����£�������ƿ��ϡ����������ڽ����Һ©��������������Ӷ��������ڼ���ϡ������������������
��ʵ��ǰ���ʽ�ζ�����Һ������ֱ�ΪV1 mL��V2 mL����������������Ϊ________ mL��
���𰸡�EDG �Ͻ���ϡ���ᷴӦ�������������壬ʹ��ƿ������ѹǿ��� ACD ���ַ�Һ©��������ѹǿ����ƿ������ѹǿ��� V1-V2
��������
��1���ٸ���ʵ��ԭ����Mg��2H��=Mg2����H2����2Al��6H��=2Al3����3H2����ͨ����ˮ������������������ָ�������״̬�£���Ͳ�еIJ���ˮ��Ҫ���������ƿ�У��������˳����A��E��D��G��
��ΪE��D��G��
�ںϽ���ϡ���ᷴӦ�����������Ҹ÷�ӦΪ���ȷ�Ӧ�������ƿ������ѹǿ����Һ�岻��˳�����䣻
��Ϊ�Ͻ���ϡ���ᷴӦ�������������壬ʹ��ƿ������ѹǿ���
��ͨ����ˮ���������������һ�����ʱ��������������ָ�������״̬���������˵�Һ�洦��ͬһˮƽ���۶���ʱ�����밼Һ�����͵��ƽ����ѡ��ACD��ȷ��
��ΪACD��
��2���ٵ���a��ͨ��ƿ�ͷ�Һ©�����������DZ��ַ�Һ©��������ѹǿ����ƿ������ѹǿ��ȣ�
��Ϊ���ַ�Һ©��������ѹǿ����ƿ������ѹǿ��ȣ�
�����������Ϊ�ⶨ��������������ռ���������ζ����ڵ�Һ��������������С�����ռ����������Ϊ(V1��V2)mL��
��Ϊ(V1��V2)mL��

����Ŀ��A��B�����л�����Ի��ܣ��й��������£�
���� | �ܶ�(g��cm-3) | �۵�/�� | �е�/�� | �ܽ��� |
A | 0.7893 | -117.3 | 78.5 | ��ˮ������Ȼ��� |
B | 0.7137 | -116.6 | 34.5 | ������ˮ |
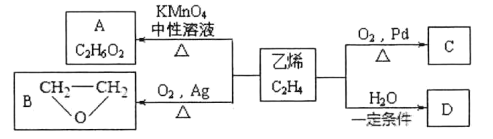
(1)Ҫ��ȥA��B�Ļ�����е�����B���ɲ��õ�_______________�����ɵõ�A��
A.���� B.�ؽᾧ C.��ȡ D.��ˮ�������Һ
(2)���л���A�����������г��ȼ�գ�A������ǡ����ȫ��Ӧ������6.72L(��״��)����������5.4gH2O��8.8gCO2��������ʵ�ʵ��ʽ��__________������ͼ��ʾ��A����Է�������Ϊ46������֪�л���A�ĺ˴Ź���������ͼ��ʾ����A�Ľṹ��ʽΪ________________��
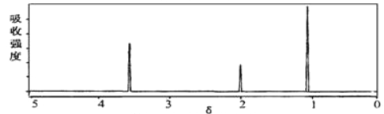
(3)��ͼ��B������ͼ��������Է�������Ϊ ________ ��
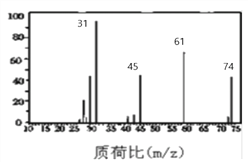
(4)B�ĺ��������ͼ��ʾ����B�Ľṹ��ʽΪ__________________________��
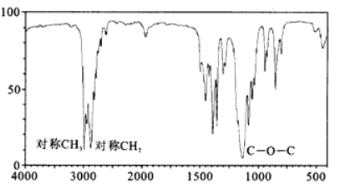
(5)ȷ��ȡһ��������A��B�Ļ����������������ȼ�գ�����������ͨ����������ˮ�Ȼ��ƺͼ�ʯ�ң����������ֱ�����14.4g��26.4g������������A��B�����ʵ���֮��_____________________��