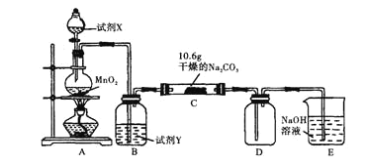
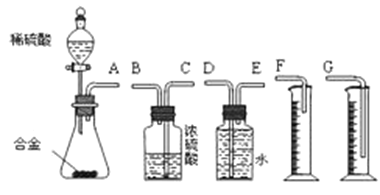
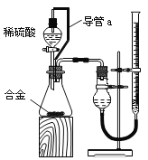
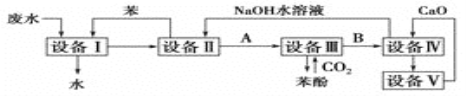
��Ŀ����
����Ŀ���������ӷ���ʽ����ȷ����
A. ����������̼������Һ��Ӧ�� 2 CH3COOH ��CO32-�� 2CH3COO����CO2����H2O
B. ������Һ������������ͭ��Ӧ�� 2CH3COOH��Cu(OH)2��Cu2+��2CH3COO����2H2O
C. ��������Һ��ͨ������������̼�� 2C6H5O����CO2��H2O��2C6H5OH��CO32-
D. ��ȩ��Һ��������������Һ����CH3CHO+2[Ag(NH3)2]++2OH-![]() CH3COO-+NH4++2Ag��+3NH3+H2O
CH3COO-+NH4++2Ag��+3NH3+H2O
���𰸡�C
������������������̼������Һ��Ӧ����CO2�����ӷ���ʽΪ2 CH3COOH ��CO32-�� 2CH3COO����CO2����H2O����A��ȷ��������Һ������������ͭ�����кͷ�Ӧ�����ӷ���ʽΪ2CH3COOH��Cu(OH)2��Cu2+��2CH3COO����2H2O����B��ȷ����������Һ��ͨ������������̼���ɱ��Ӻ�̼�����ƣ����ӷ���ʽΪ��C6H5O����CO2��H2O��C6H5OH��HCO3- ����C������ȩ��Һ��������������Һ���ȣ����������������ӷ���ʽΪCH3CHO+2[Ag(NH3)2]++2OH-![]() CH3COO-+NH4++2Ag��+3NH3+H2O����D��ȷ��
CH3COO-+NH4++2Ag��+3NH3+H2O����D��ȷ��

����Ŀ�������������Ǵ�������Ҫ��Ⱦ��ǵ�ǰ������������Ҫ�о�����֮һ��
��1��������ԭ����������
��N2(g)+O2(g)=2NO(g) ��H1
��4NH3(g)+3O2(g)=2N2(g)+6H2O(l) ��H2
��4NH3(g)+6NO(g)=5N2(g)+6H2O(1) ��H=_______________��(�ú���H1����H2��ʽ�ӱ�ʾ)
���ݷ�Ӧ�ڣ��ɽ�����ֱ������ȼ�ϵ�أ���KOH��Һ���������Һ��д�������缫��Ӧʽ��______________________________________________________��
��2��һ���¶��£���2L�����ܱ������г���4.0mol NO2��4.0mol CO���ڴ��������·�����Ӧ4CO(g)+2NO2(g) ![]() N2(g)+4CO2(g) ��H<0���������������£�
N2(g)+4CO2(g) ��H<0���������������£�
0min | 5min | 10min | 15min | 20min | |
c(NO2)/mol/L | 2.0 | 1.7 | 1.56 | 1.5 | 1.5 |
c(N2)/mol/L | 0 | 0.15 | 0.22 | 0.25 | 0.25 |
��0��5min����NO2��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ_________________��
�����±�����˵���÷�Ӧ���ﵽƽ��״̬����________��
A��������ɫ���ٱ仯 B�������ܶȲ��ٱ仯 C������ƽ����Է����������ٱ仯
��20 minʱ�������¶Ȳ��䣬������������м���l.0mol NO2��l.0molCO����t1ʱ��Ӧ�ٴδﵽƽ�⣬��NO2��ת���ʱ�ԭƽ��_______������������������С���������䣩��
�ܸ��¶��·�Ӧ�Ļ�ѧƽ�ⳣ��K= _________________��
��3��ʪ������������NaClO2 ��Һ��Ϊ���ռ��ɶ���������������323 K �£�����������NaClO2 ��Һ��ͨ�뺬NO ����������ַ�Ӧ����Һ������Ũ�ȵķ���������±���
���� | NO3- | NO2- | Cl- |
c/(mol L-1) | 2.0��10-4 | 1.0��10-4 | 1.75��10-4 |
���ݱ������ݣ�д��NaClO2��Һ���������з����ܷ�Ӧ�����ӷ���ʽ______________________��
����Ŀ����I��������Ԫ��W��X��Y��Z�����ڱ��е����λ�������ʾ��������Ԫ��ԭ�ӵ�����������֮��Ϊ21���ش��������⣺
W | X | |||
Y | Z |
��1��Z��Ԫ�����ڱ��е�λ��______________��
��2��X������⻯��ĵ���ʽΪ______��
��3��Y������NaOH��Һ��Ӧ�����ӷ���ʽΪ��________����ҵ����Y���ʵĻ�ѧ����ʽΪ__________��
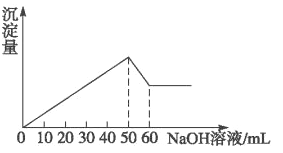
��4����ʢ��3 mL��������Һ���Թ�����뼸��W������������ˮ����Ũ��Һ��ʵ������Ϊ_________��
��II���״�����Ҫ�Ļ���ԭ�ϣ��ֿ���Ϊȼ�ϡ����úϳ�������Ҫ�ɷ�ΪCO��CO2��H2���ڴ����������ºϳɼ״�������������Ӧ���£�
��CO��g����2H2��g��![]() CH3OH��g����H1
CH3OH��g����H1
��CO2��g����3H2��g��![]() CH3OH��g����H2O��g����H2
CH3OH��g����H2O��g����H2
��CO2��g����H2��g��![]() CO��g����H2O��g����H3
CO��g����H2O��g����H3
�ش��������⣺��֪��Ӧ���е���صĻ�ѧ���������������
��ѧ�� | H��H | C��O |
��CO�еĻ�ѧ���� | H��O | C��H |
E/��kJ��mol��1�� | 436 | 343 | 1076 | 465 | 413 |
��5���ɴ˼��㦤H1��__kJ��mol��1����֪��H2����58kJ��mol��1����H3��__kJ��mol��1��