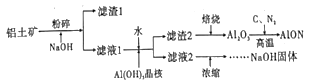
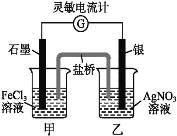
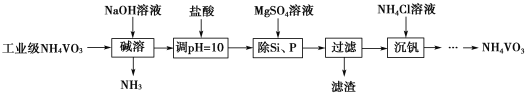
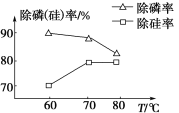
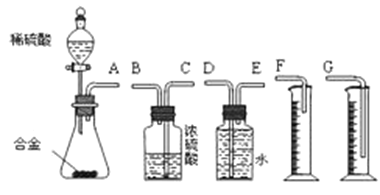
��Ŀ����
����Ŀ����ϩ�ڲ�ͬ�������¿ɱ������ɲ�ͬ�����A��B��C����֪��ȡ0.01mol A������������ȫ��Ӧ������224mL(��״��)���塣C��B��ͬ���칹�壬C�����Ƶ�������ͭ��Һһ����ȣ��������ɫ������
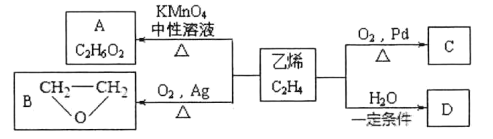
��֪��һ��̼ԭ�������ж���-OH�����Dz��ȶ��Ľṹ��
(1)C�к��еĹ�����������______����ϩת��ΪD�ķ�Ӧ����______��
(2)д��D��������Ӧ����C�Ļ�ѧ����ʽ______��
(3)����˵����ȷ����______��
A. B�����е�����ԭ����ͬһƽ����
B. һ�������£�B����CO2�ۺ����ɿɽ���ĸ߷���
C. ��ϩ��һ�������±���������Ҳ����������
D. A��D��ͬϵ��
���𰸡�ȩ�� �ӳɷ�Ӧ 2CH3CH2OH+O2![]() 2CH3CHO+2H2O BC
2CH3CHO+2H2O BC
��������
0.01molA�������Ľ�������ȫ���ú�����224mL(��״��)���壬���������������ʵ���n(H2)=![]() =0.01mol�����A�ķ���ʽ��֪��A�����к���2���ǻ�����AΪ�Ҷ������ṹ��ʽΪHOCH2CH2OH��C��B��ͬ���칹�壬C�����Ƶ�������ͭ��Һһ����ȣ��������ɫ��������C����ȩ����CΪCH3CHO����ϩ��ˮ�����ӳɷ�Ӧ����DΪCH3CH2OH���Դ������
=0.01mol�����A�ķ���ʽ��֪��A�����к���2���ǻ�����AΪ�Ҷ������ṹ��ʽΪHOCH2CH2OH��C��B��ͬ���칹�壬C�����Ƶ�������ͭ��Һһ����ȣ��������ɫ��������C����ȩ����CΪCH3CHO����ϩ��ˮ�����ӳɷ�Ӧ����DΪCH3CH2OH���Դ������
��������������֪��A��HOCH2CH2OH��CΪCH3CHO��DΪCH3CH2OH��
(1)CΪCH3CHO������������Ϊȩ������ϩCH2=CH2��H2O��һ�������·����ӳɷ�Ӧ����D��CH3CH2OH������Cת��ΪD�ķ�Ӧ����Ϊ�ӳɷ�Ӧ��
(2)D��CH3CH2OH���Ҵ������к��д��ǻ�����������Cu��Ag���£����ȷ���������Ӧ����C����Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
(3)A.B�ǻ������飬���ʷ�����Cԭ�Ӿ�Ϊ����Cԭ�ӣ����Cԭ�����ӵ�ԭ�ӹ��ɵ���������ṹ������ԭ�Ӳ�����ͬһƽ���ϣ�A����
B.һ�������£�B����CO2�ۺ����ɿɽ���ĸ߷���Ϊ�����ᣬB��ȷ��
C.��ϩ�����к�̼̼˫������һ�������±���������Ҳ���������ᣬC��ȷ��
D.A ���Ҷ�����D���Ҵ���A��D�����к�-OH��Ŀ��ͬ���Ҵ����߲���ͬϵ�D���� �ʺ���ѡ����BC��