��Ŀ����
7��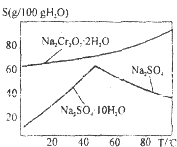 ��ҵ���Ը�������Ҫ�ɷ�FeO•Cr2O3����̼���ơ�����������Ϊԭ�������ظ����ƣ�Na2Cr2O7•2H2O������Ҫ��Ӧ���£�
��ҵ���Ը�������Ҫ�ɷ�FeO•Cr2O3����̼���ơ�����������Ϊԭ�������ظ����ƣ�Na2Cr2O7•2H2O������Ҫ��Ӧ���£���4FeO•Cr2O3+8Na2CO3+7O2$\frac{\underline{\;����\;}}{\;}$8Na2CO3+2Fe2O3+8CO2
��2Na2Cro4+H2SO4?Na2SO4+Na2Cr2O7+H2O
��1����ҵ�Ϸ�Ӧ���費�Ͻ��裬��Ŀ����ʹ��Ӧ���ֽӴ����ӿ췴Ӧ���ʣ�
��2����ͼ�Ǻ췯�ƣ�Na2Cr2O7•2H2O����Na2SO4���ܽ�����ߣ�
��Na2Cr2O7Na��Na2SO4�Ļ����Һ����ȡNa2Cr2O7����IJ������Ƚ������Һ����Ũ�������ȹ��ˣ�Ȼ����Һ��ȴ�ᾧ���Ӷ������췯�ƣ����ȹ��˵�Ŀ���������������ƽᾧ���������Է�ֹNa2Cr2O7•2H2O�ᾧ������
��3����Cr2O72-�ķ�ˮҪ������ѧ������ʹc��Cr2O72+������5.0��10-5mol/L���²����ŷţ��ó����������÷�Һ�Ƿ���֮һ��
��֪��Ksp��BaCr2O7��=2��10-7����c��Ba2+������Ӧ�ﵽ4��10-3mol/L��
��4�����ظ���زⶨ����ʯ�����ĺ�����ʵ�鲽�����£�
����1����mg����ʯ��Ũ��������ܽ�
����2������SnCl2��Һ��Fe3+��ԭ
����3����������Һ��ȴ������HgCl2��Һ����������Sn2+����ΪSn4+
����4������15mL���������Ļ���ἰ5��0.2%������������ָʾ��
����5��������cmol-1�ظ������Һ�ζ�����Һ���ȶ���ɫ����Ϊ�յ㣬�����ظ������VmL
����֪��6Fe2++Cr2O72-+14H+�T6Fe3++2Cr3++7H2O��
��д��SnCl2��ԭFe3+�����ӷ���ʽ2Fe3++Sn2+=Sn4++2Fe2+��
����mg����ʯ�к���Ԫ�ص��������ú���ĸc��V�Ĵ���ʽ��ʾ��0.336cVg��
����ʡȥ����ۣ������ⶨ�����ĺ���ƫ�ߣ��ƫ�ߡ�����ƫ�͡�����Ӱ�족����
�ܲ���5ʹ�õIJ�����������ʽ�ζ��ܡ���ƿ��
���� ��1����Ӧ�����ڻ�תҤ�н��У���Ӧʱ�費�Ͻ��裬ʹ��Ӧ���ֽӴ����ӿ췴Ӧ���ʣ�
��2����ͼ��֪���¶ȸ������������ƽᾧ���������Է�ֹNa2Cr2O7•2H2O�ᾧ������
��3��c��Cr2O72+��С��5.0��10-5mol/L���ܴ�꣬���Ksp��BaCr2O7��=2��10-7����������ӵ���СŨ�ȣ�
��4����SnCl2��ԭFe3+�����������ӣ�����������ΪSn4+��
�ڸ���n=cV����������ظ�������ӵ����ʵ������ٽ�Ϸ�Ӧ����ʽ�������Ʒ�к��������ӵ����ʵ�����������m=nM�������Ԫ�ص�������
���ظ������Һ������Sn2+��ʹ���ĵ��ظ������Һ���ƫ������Ԫ�ص�����ƫ�ߣ�
�ܲ���5�IJ����ǵζ������Ը�����ؾ���ǿ�����ԣ�Ӧʹ����ʽ�ζ��ܣ���ƿ��ʢ�Ŵ���Һ��
��� �⣺��1����Ӧ�����ڻ�תҤ�н��У���Ӧʱ�費�Ͻ��裬ʹ��Ӧ���ֽӴ����ӿ췴Ӧ���ʣ�
�ʴ�Ϊ��ʹ��Ӧ���ֽӴ����ӿ췴Ӧ���ʣ�
��2����ͼ��֪���¶ȸ������������ƽᾧ���������Է�ֹNa2Cr2O7•2H2O�ᾧ��������Һ���¶Ƚϸߣ�����Һ�л�þ�����Ҫ��ȴ�ᾧ��
�ʴ�Ϊ�������������ƽᾧ���������Է�ֹNa2Cr2O7•2H2O�ᾧ������
��3��c��Cr2O72+��С��5.0��10-5mol/L���ܴ�꣬��֪Ksp��BaCr2O7��=2��10-7����c��Ba2+����$\frac{2��1{0}^{-7}}{5��1{0}^{-5}}$mol/L=4��10-3mol/L��
�ʴ�Ϊ��4��10-3mol/L��
��4����SnCl2��ԭFe3+�����������ӣ�����������ΪSn4+����Ӧ���ӷ���ʽΪ��2Fe3++Sn2+=Sn4++2Fe2+��
�ʴ�Ϊ��2Fe3++Sn2+=Sn4++2Fe2+��
��VmL cmol/l���ظ���ص����ʵ���Ϊ��cmol/L��V��10-3L=cV��10-3mol�����ݷ�Ӧ6Fe2++Cr2O72-+14H+�T6Fe3++2Cr3++7H2O��֪��mg��Ʒ�к�����Ԫ�ص�����Ϊ��56g/mol��6��cV��10-3mol=0.336cVg��
����0.336cVg��
���ظ������Һ������Sn2+��ʹ���ĵ��ظ������Һ���ƫ������Ԫ�ص�����ƫ�ߣ����ⶨ�����ĺ���ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
�ܲ���5�IJ����ǵζ������Ը�����ؾ���ǿ�����ԣ�Ӧʹ����ʽ�ζ��ܣ���ƿ��ʢ�Ŵ���Һ��
�ʴ�Ϊ����ʽ�ζ��ܡ���ƿ��
���� ���⿼��̽��������ɡ��������ʺ����ķ�������Ŀ�Ѷ��еȣ��漰���û�ѧ����ı�ʾ������������ԭ��Ӧ�ζ�����ѧ�����֪ʶ���Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ��������ʵ�Ļ���������֪ʶ������������������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | 2.24 L CO2�к��е�ԭ����Ϊ0.3��6.02��1023 | |
| B�� | 0.1 L 3 mol•L-1��NH4NO3��Һ�к��е�NH4+��ĿΪ0.3��6.02��1023 | |
| C�� | 5.6 g���������ᷴӦʧȥ�ĵ�����һ��Ϊ0.3��6.02��1023 | |
| D�� | 4.5 g SiO2�����к��еĹ�������ĿΪ0.3��6.02��1023 |
| A�� | NaCl | B�� | H2SO4 | C�� | AgNO3 | D�� | Na2SO4 |
| A�� | ���۵⻯����Һ�ڿ����б�����4I+O2+2H2O�T4OH-+2I2 | |
| B�� | ��NaOH��Һ����������2OH-+2Cl2�T2Cl-+H2O | |
| C�� | ����CO2ͨ�뱽������Һ�У�2C6H5O-+CO2+H2O��2C6H5OH+CO32- | |
| D�� | �Ȼ�þ��Һ�백ˮ��Ӧ��Mg2++2OH-�TMg��OH��2�� |
| A�� | �����ǵķ���ʽ��C6H12O6 | B�� | �������ܷ���������Ӧ | ||
| C�� | ��������������Ҫ��������Դ | D�� | ��������������ͬ���칹�� |
| A�� | 1��N��N�����ѵ�ͬʱ����6��N-H���γ� | |
| B�� | ���������ܶȲ��ٸı� | |
| C�� | v����H2��=$\frac{3}{2}$ v����NH3�� | |
| D�� | N2��H2��NH3�������Ϊ1��3��2 |
| A�� | ��̬�⻯����ȶ��ԣ�H2O��H2S��SiH4 | |
| B�� | ���Ӱ뾶��Cl-��O2-��Mg2+ | |
| C�� | �������ԣ�H3PO4��H2SO4��HClO4 | |
| D�� | �۵㣺KCl��KI��K |
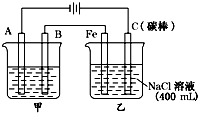 ��ͼΪ������ļ��������أ��Իش�
��ͼΪ������ļ��������أ��Իش�