��Ŀ����
17�������ӵ�����ԼΪ6.02��1023mol-1������������ȷ���ǣ�������| A�� | 2.24 L CO2�к��е�ԭ����Ϊ0.3��6.02��1023 | |
| B�� | 0.1 L 3 mol•L-1��NH4NO3��Һ�к��е�NH4+��ĿΪ0.3��6.02��1023 | |
| C�� | 5.6 g���������ᷴӦʧȥ�ĵ�����һ��Ϊ0.3��6.02��1023 | |
| D�� | 4.5 g SiO2�����к��еĹ�������ĿΪ0.3��6.02��1023 |
���� A��û������������������Ħ�������ʽ���㣻
B��NH4+��ˮ��Һ�в���ˮ�⣻
C��5.6�˵��������ᷴӦʧȥ�ĵ��Ӳ�һ������0.3mol�������۹���ʱΪ0.2mol��
D��4.5g SiO2 ����0.075mol��ÿ��Si��4��Oԭ�ӳɼ��������״�ṹ������1molSiO2��4mol��������
��� �⣺A�������2.24LCO2�к��е�ԭ������Ϊ0.3��6.02��1023������ûָ����ʲô�����£���A����
B��NH4+��ˮ��Һ�в���ˮ�⣬���0.1L3mol•L-1��NH4NO3��Һ�к��е�NH4+��Ŀ����0.3��6.02��1023����B����
C��5.6�˵��������ᷴӦʧȥ�ĵ��Ӳ�һ������0.3mol�������۹���ʱΪ0.2mol���������ʱFe���Ⱥ����ᷴӦ����Fe3+����Fe3+�뵥������Ӧ����Fe2+�������ܷ�Ӧ�������Fe2+��ʧȥ����������0.3mol����C����
D��4.5g SiO2 ����0.075mol��ÿ��Si��4��Oԭ�ӳɼ��������״�ṹ������1molSiO2��4mol ��������4.5gSiO2���еĹ�������ĿΪ0.3��6.02��1023����D��ȷ��
��ѡD��
���� ���⿼�鰢���ӵ�����������������ʵ����ʵ����ǽ���Ĺؼ����ѶȲ���

��ϰ��ϵ�д�
 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�
�����Ŀ
20��ʯ�ͻ���ר���ɶ���Ժʿ��2007��ȹ�����߿�ѧ������������ʯ�ͻ��������������µ������ߺ���ɫ��ѧ�Ŀ����ߣ������ƵĶ���ʯ�����ƴ�������ؽ������ҹ�ʯ�ͻ�����Ʒ�ijɱ�������˵������ȷ���ǣ�������
| A�� | ����IJ����Ǻ���һ������ʯ�ͻ���ˮƽ��չ�ı�־ | |
| B�� | ʯ���ѻ�����ҪĿ����Ϊ�˻�ö����IJ������� | |
| C�� | �����ն����γ��뻯ʯȼ��ú��ʯ�͵Ĵ���ʹ���� | |
| D�� | ʯ�͵���Ҫ�ɷ���̼�⻯���� |
8��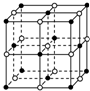 ʳ�ξ������������ӣ�ͼ�еġ�
ʳ�ξ������������ӣ�ͼ�еġ� �����������ӣ�ͼ�еġ��𡱣���ɵģ��Ҿ�Ϊ�Ⱦ���Ľ������У���֪ʳ�ε��ܶ���2.2g•cm-3�������ӵ�����6.02��1023 mol-1����ʳ�ξ���������������������������ļ�ľ�����ӽ��ڣ�������
�����������ӣ�ͼ�еġ��𡱣���ɵģ��Ҿ�Ϊ�Ⱦ���Ľ������У���֪ʳ�ε��ܶ���2.2g•cm-3�������ӵ�����6.02��1023 mol-1����ʳ�ξ���������������������������ļ�ľ�����ӽ��ڣ�������
 ʳ�ξ������������ӣ�ͼ�еġ�
ʳ�ξ������������ӣ�ͼ�еġ� �����������ӣ�ͼ�еġ��𡱣���ɵģ��Ҿ�Ϊ�Ⱦ���Ľ������У���֪ʳ�ε��ܶ���2.2g•cm-3�������ӵ�����6.02��1023 mol-1����ʳ�ξ���������������������������ļ�ľ�����ӽ��ڣ�������
�����������ӣ�ͼ�еġ��𡱣���ɵģ��Ҿ�Ϊ�Ⱦ���Ľ������У���֪ʳ�ε��ܶ���2.2g•cm-3�������ӵ�����6.02��1023 mol-1����ʳ�ξ���������������������������ļ�ľ�����ӽ��ڣ�������| A�� | 3.0��10-8 cm | B�� | 3.5��10-8 cm | C�� | 4.0��10-8 cm | D�� | 5.0��10-8 cm |
5����һ�������£���Ҫʹ��ӦCO��g��+NO2��g��?CO2��g��+NO��g���ķ�Ӧ��������ɲ�ȡ�Ĵ�ʩ�У�������
| A�� | �����¶� | B�� | ����������� | C�� | �����¶� | D�� | ѡ�ø�Ч���� |
12������NA��ʾ�����ӵ�����������������ȷ���ǣ�������
| A�� | 1 mol Fe������������Ӧʱת�Ƶĵ�����Ϊ2NA | |
| B�� | 0.2 mol S�ڿ����г��ȼ�գ�ת�Ƶ�����Ϊ0.6NA | |
| C�� | 0.1molCl2����ˮ�з�����Ӧ��ת�Ƶ�����Ϊ0.1NA | |
| D�� | 1.5 mol Na2O2�������Ķ�����̼��ַ�Ӧ��ת�Ƶ�����Ϊ1.5NA |
2�����з�Ӧ�У�������ȡ����Ӧ���ǣ�������
| A�� | ���еμ�Ũ��ˮ | B�� | �ױ���Ũ���ᡢŨ�����Ϲ��� | ||
| C�� | ������������Ϻ���� | D�� | �Ҵ���Ũ�����ϼ�����170�� |
9������ת����ϵ�ж�����˵����ȷ���ǣ�������


| A�� | ��Ӧ�۵õ������������к����Ҵ������ᣬ���ñ�������������Һ��ȥ | |
| B�� | ��Ӧ���У�1mol�����ǿ�����3mol�Ҵ� | |
| C�� | ���ڿ��������ƺ��ͭ˿���Ȳ����Ҵ��пɵõ����� | |
| D�� | ��Ӧ���У���C6H10O5��n�ɱ�ʾ���ۻ���ά�� |
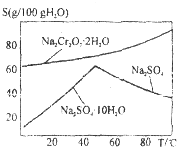 ��ҵ���Ը�������Ҫ�ɷ�FeO•Cr2O3����̼���ơ�����������Ϊԭ�������ظ����ƣ�Na2Cr2O7•2H2O������Ҫ��Ӧ���£�
��ҵ���Ը�������Ҫ�ɷ�FeO•Cr2O3����̼���ơ�����������Ϊԭ�������ظ����ƣ�Na2Cr2O7•2H2O������Ҫ��Ӧ���£�