��Ŀ����
17��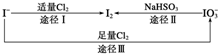 �������������������֮һ�ش������йص�����⣺
�������������������֮һ�ش������йص�����⣺��1�����ڵؿ�����Ҫ��NaIO3����ʽ���ڣ��ں�ˮ����Ҫ��I-����ʽ���ڣ���������֮���ת����ϵ��ͼ��ʾ��
����˵���в���ȷ����C
A���õ���KI��ֽ��ʳ����ӵ���ʱ����KI��ֽ�����
B������Cl2��ʹʪ��ġ��ѱ����ĵ���KI��ֽ��ɫ��ԭ�������5Cl2+I2+6H2O�T2HIO3+10HCl
C����ͼ��֪�����Ե�ǿ��˳��ΪCl2��I2��IO3-
D��;������������1mol I2����Ӧ��ת�Ƶĵ�����Ϊ10NA
��2���ⲻ������ˮ���������ڵ⻯����Һ�����ɺ������ӣ�aq��ʾˮ��״̬��
I2��aq��+I-��aq���TI3-��aq�� ����
I2��aq��+2I-��aq���TI42-��aq�� ����
��Ӧ�ڵ�ƽ�ⳣ������ʽ��K=$\frac{c��{I}_{4}^{2-}��}{c��{I}_{2}��{c}^{2}��{I}^{-}��}$��
I2����ʼŨ�Ⱦ�Ϊ0.1mol•L-1����ͬ��ʼŨ��KI��Һ�У��ﵽƽ��ʱ�������ӵ����ʵ����������±���
| KI��ʼŨ��/mol•L-1 | 0.02 | 0.04 | 0.06 | 0.08 | 0.10 |
| I3-% | 88.98 | 88.04 | 85.28 | 82.26 | 79.23 |
| I42-% | 4.38 | 8.67 | 12.60 | 16.21 | 19.53 |
��3��ijѧϰС���á���ӵ��������ⶨ����CuCl2•2H2O�������������������I-������Ӧ�������������ʣ��Ĵ��ȣ��������£�ȡ0.36g��������ˮ���������KI���壬��ַ�Ӧ�����ɰ�ɫ��������0.1000mol•L-1 Na2S2O3����Һ�ζ�������ζ��յ�ʱ������Na2S2O3����Һ20.00mL��
�ٿ�ѡ�õ�����ָʾ�����ζ�ָʾ�����ζ��յ����������Һ����ɫ��Ϊ��ɫ�Ұ�����ڲ��ָ�Ϊ��ɫ��
��CuCl2��Һ��KI��Ӧ�����ӷ���ʽΪ2Cu2++4I-=2CuI��+I2����
�۸�������CuCl2•2H2O�������ٷ���Ϊ95%��
���� ��1��A����������NaIO3��KI��Ӧ���ɵⵥ�ʣ�����۱���ɫ��B���������۵��۵⻯����ֽ����ɫ��ʪ������������������������5Cl2+I2+6H2O�T2HIO3+10HCl�����ⷴӦ������ɫ��ȥ��C����������ԭ��Ӧ�У��������������Դ�����������������ԣ�������˳��ΪCl2��IO3-��I2��DIO3-�е�Ļ��ϼ�Ϊ+5�ۣ�I2�еĻ��ϼ�Ϊ0�ۣ�����˫ԭ�ӷ��ӣ�û����1mol I2��ת�Ƶĵ�����Ϊ10NA��
��2�����ݻ�ѧƽ�ⳣ���ĸ�����ݻ�ѧ����ʽI2��aq��+2I-��aq���TI42-��aq����д��ƽ�ⳣ���ı���ʽ������KI��Ũ�ȵ����ӣ�I3-%��ֵ�����I42-%��ֵʼ�մ��ھ��Ե����ƣ���������KI��Ũ�ȵ����ӣ�I42-%��ֵ�����ӣ�
��3���������Һ�м��������Һ����Һ����ɫ��������Һ�м���Na2S2O3��Һ�����ڵⱻNa2S2O3��ԭΪ�����ӣ���Һ��ɫ����ȥ������Һ��ɫ�պ���ʧʱ����������ȫ��Ӧ���Ӷ����еζ��յ���ж���
��CuCl2��Һ��KI��Һ����ʱ������������ԭ��Ӧ��+2�۵�ͭ���ӵõ��ӣ���Ϊ+1�ۣ�-1������ʧ���ӱ�Ϊ0�ۣ����ӷ�Ӧ����ѭ���ӡ�����غ㣻
�۸���������ԭ��Ӧ���������õ��ĵ��ӵ������뻹ԭ��ʧȥ�ĵ��ӵ�������ȣ�����CuCl2��Һ�м���KI������ʧȥ���Ӹ���+2�۵�ͭ�������ӱ�����Ϊ�ⵥ�ʣ�����Na2S2O3��Һ������+2�۵�����ʧ���ӱ���õ���ɵ����ӣ��൱��+2�۵���ֱ��ʧ���ӱ�+2�۵�ͭ�õ�����Ϊ+1�ۣ��ó����¹�ϵ��CuCl2•2H2O��Na2S2O3����n��CuCl2•2H2O��=n��Na2S2O3��=0.002mol������m��CuCl2•2H2O��=0.002mol��171gmol-=0.342g���Դ˼���������CuCl2•2H2O�������ٷ�����
��� �⣺��1��A������������NaIO3��KI����������ԭ��Ӧ��-1�۵ĵ����ӱ�����Ϊ�ⵥ�ʣ����������۱���ɫ����A����
B��ʪ��ġ��ѱ����ĵ���KI��ֽ��ɫ˵���е����Ӵ����������������ֱ��Ϊ��ɫ�������ڷ�������������������ԭ��Ӧ���䷴Ӧ����ʽΪ��5Cl2+I2+6H2O�T2HIO3+10HCl�����ʵ���ʧ����ɫ��ʧ����B����
C����ͼ��֪�����ܽ�����������Ϊ�⣬����������ܽ�����������Ϊ���ʵ⣬����������ԭ��Ӧ���������������Դ�����������������ԣ�����Cl2��IO3-��I2����C��ȷ��
D����Ӧ����IO3-��HSO32-����������ԭ��Ӧ��+5�۵ĵ�õ������ɵⵥ�ʣ�ÿһĦ����ԭ�ӵõ�5mol���ӣ��ⵥ����˫ԭ�ӷ��ӣ�����ÿ����1mol�ⵥ����Ҫ��10mol�����������1molI2ת��10mol���ӣ���D����
�ʴ�Ϊ��C��
��2���ɻ�ѧ��Ӧ����ʽI2��aq��+2I-��aq���TI42-��aq������֪��ѧƽ�ⳣ���ı���ʽΪK=$\frac{c��{I}_{4}^{2-}��}{c��{I}_{2}��{c}^{2}��{I}^{-}��}$��ͨ���۲����б��е����ݣ���KI����ʼŨ�ȸı�ʱI3-%��I42-%������Ҳ������Ӧ�ĸı䣬��KI����ʼŨ����ô�ı䣬ƽ����ϵ��I3-%�����I42-%�����о��Ե����ƣ���������KI����ʼŨ�ȵ����ӣ�I3-%�Ǽ�С�ģ�
�ʴ�Ϊ��K=$\frac{c��{I}_{4}^{2-}��}{c��{I}_{2}��{c}^{2}��{I}^{-}��}$����������ͬʱ��I3-���ӵ����ʵ����������ʼ��ռ�������ƣ���I3-�����ߣ�I42-�����ͣ�������I-����Ũ�ȵ����ӣ�I3-���ӵ����ʵ����������ͣ�I42-���ӵ����ʵ����������ӣ�
��3���������Һ�м��������Һ����Һ����ɫ��������Һ�м���Na2S2O3��Һ�����ڵⱻNa2S2O3��ԭΪ�����ӣ���Һ��ɫ����ȥ����ѡ�����Ϊָʾ�����۲쵽��Һ����ɫ��Ϊ��ɫ�Ұ�����ڲ��ָ�Ϊ��ɫ��Ϊ�ζ��յ㣬
�ʴ�Ϊ��������ָʾ���� ��Һ����ɫ��Ϊ��ɫ�Ұ�����ڲ��ָ�Ϊ��ɫ��
��CuCl2��Һ��KI��Һ����ʱ������������ԭ��Ӧ��+2�۵�ͭ���ӵõ��ӣ���Ϊ+1�ۣ�-1������ʧ���ӱ�Ϊ0�ۣ��ɵ��ӡ�����غ��֪���ӷ�ӦΪ2Cu2++4I-=2CuI��+I2��
�ʴ�Ϊ��2Cu2++4I-=2CuI��+I2��
��0.36gCuCl2•2H2O��+2�۵�ͭ���ӵõ��ӱ���ԭΪ+1�۵���ͭ���ӣ������ӱ���ԭΪ�ⵥ�ʣ��������۱���ɫ��ѡ�������Ϊָʾ������Ӧ�����ϵ�м���0.1000mol•L-1 Na2S2O3����Һ20mLʱ��Һ�б�Ϊ��ɫ˵�����ɵĵ��ʵ��б�����Ϊ�����ӣ���Ӧ���£�I2+2S2O32-�TS4O62-+2I-���൱��Na2S2O3��+4�۵���ʧȥ����ֱ�Ӹ���+2�۵�ͭ���ٸ���2Cu2++4I-=2CuI��+I2������CuCl2•2H2O��Na2S2O3��n��Na2S2O3��=0.002mol����Ӧʱ1molNa2S2O3ʧȥ1mol���ӣ�����n��CuCl2•2H2O��=0.002mol����m��CuCl2•2H2O��=0.342g������������CuCl2•2H2O�������ٷ���Ϊ$\frac{0.342g}{0.36g}��$100%=95%���ʴ�Ϊ��95%��
���� �������ʺ����IJⶨ�����ʵ����ʣ�Ϊ��Ƶ���㣬�������е���Ϣ���������ԭ��Ӧ����ѧƽ�ⳣ����Ԫ�ػ���������ʡ�����������֪ʶΪ���Ĺؼ�����Ŀ�ۺ��Խ�ǿ����Ŀ�Ѷ��еȣ�������ԭ��Ӧ����ϵʽ�ļ���Ϊ�����ѵ㣮

 ��У����ϵ�д�
��У����ϵ�д�| A�� | �٢ۢ� | B�� | �ڢۢ� | C�� | �٢ڢ� | D�� | �ڢܢ� |
| W | X | |
| Y | Z |
| A�� | X��Y��Z������⻯���ȶ�����������Y | |
| B�� | ZԪ������������Ӧˮ���������һ��ǿ��Y | |
| C�� | X��YԪ���γɵ����ֳ���������ֱ�����ˮ����ҺPH��7 | |
| D�� | ZԪ�ص����ڻ�ѧ��Ӧ��ֻ���������� |
| A�� | 1mol FeCl3��ȫת��ΪFe��OH��3������γ�NA������ | |
| B�� | 1 L 0.1 mol/L Na2CO3��Һ��CO32-��HCO3-����Ϊ0.1 NA | |
| C�� | ��״���£�22.4L NO��11.2L O2��Ϻ�����ķ�������ΪNA | |
| D�� | 0.2 mol��Ȳ����ȩ�����������������ȫȼ�գ����������ķ�����Ϊ0.5NA |
| A�� | ������������������Һ��Ӧ��Al2O3+2OH-=2AlO2-+H2O | |
| B�� | ������Һ������������Һ��Ӧ��SO42-+Ba2+=BaSO4�� | |
| C�� | ̼��Ƹ����ᷴӦ��CO32-+2H+=H2O+CO2�� | |
| D�� | �Ȼ����м���������������ƣ�Al3++3OH-�TAl��OH��3�� |
| A�� | �ֻ����õ�����ӵ�����ڶ��ε�� | |
| B�� | Ǧ���طŵ�ʱ��������������С | |
| C�� | ����ȼ�ϵ�ؿɰѻ�ѧ��ת��Ϊ���� | |
| D�� | п�̸ɵ���У�п�缫�Ǹ��� |
| A�� | bһ��С��c | B�� | ���ʵ�������Z��Y | ||
| C�� | Y2-�Ļ�ԭ�Դ���Z- | D�� | X��Y�ɴ���ͬ���ڻ�X��Y�������� |
 �������������A��Ϊͬ���칹�壮
�������������A��Ϊͬ���칹�壮