��Ŀ����
7�����к͵ζ�������ij�ռ�Ĵ��ȣ��Ը���ʵ�鲽����д���пհף���1�����ƴ�����Һ����4.21g���������������ᷴӦ�����ʵ�NaOH�������250.00mL����Һ�����õ����������ձ�������������ͷ�ιܡ�250mL ����ƿ��
��2����0.4mol•L-1�ı�������Һע��ྻ����ʽ�ζ����У�ʹҺ��̶��ڡ�0�����ϣ��̶���������ת����ʹ�ζ��ܼ��첿�ֳ�����Һ��Ȼ�����Һ�汣����0�̶Ȼ�0�̶����£����¶������ü�ʽ�ζ�������ƿ�м���20.00mL������Һ�����Ӽ��μ�����ָʾ����
��3��ʵ������
| ������ | ����Һ�������mL�� | ����0.4000mol•L-1����������mL�� | |
| �ζ�ǰ��mL�� | �ζ���mL�� | ||
| 1 | 20.00 | 2.10 | 22.00 |
| 2 | 20.00 | 0.90 | 21.00 |
��4�������������ζ�ǰ������ʽ�ζ��ܶ������յ������ȷ�����ý��ƫ�ͣ�ƫ�ߡ�ƫ�ͣ�
���� ��1����������һ�����ʵ���Ũ�ȵ���Һʹ�õ��������
��2���ζ��ܡ�0���̶�����û�п̶ȣ�
��3�����ݱ��еζ����ݼ�������ı�Һ��ƽ�������Ȼ�����c�����⣩=$\frac{c��������V������}{V�����⣩}$���������Һ��Ũ�ȣ������250.00mL����Һ��NaOH�����ʵ�����������Ȼ�����������
��4������ʵ�������c�����⣩=$\frac{c��������V������}{V�����⣩}$��Ӱ�������������
��� �⣺��1������250ml����Һ����Ҫʹ�õ������У��ձ�������������ͷ�ιܡ�250mL����ƿ��
�ʴ�Ϊ��250mL����ƿ��
��2���ζ��ܡ�0���̶�����û�п̶ȣ�����Ҫ����Һ�汣����0�̶Ȼ�0�̶����£��������������ĵı���Һ�������
�ʴ�Ϊ��0�̶Ȼ�0�̶����£�
��3������������������ֱ�Ϊ��22.00mL-2.10mL=19.90mL��21.00mL-0.90mL=20.10mL�����εζ����ݶ�����Ч�ģ��������������ƽ�����Ϊ��$\frac{19.90mL+20.10mL}{2}$=20.20mL��
c�����⣩=$\frac{c��������V������}{V�����⣩}$=$\frac{0.4000��0.0200}{0.0200}$=0.4000mol•L-1��
����250.00mL������Һ���У�m���ռ�TcV•M�T0.4000mol•L-1��0.25L��40g/mol=4g��
�ռ�Ĵ��Ȧأ��ռ=$\frac{4g}{4.21g}$��100%=95%��
�ʴ�Ϊ��95%��
��4�����ζ�ǰ������ʽ�ζ��ܶ����������ƫ���յ������ȷ�������ζ����IJ�ƫС������Һ�������С������c�����⣩=$\frac{c��������V������}{V�����⣩}$��֪�����������ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
���� ���⿼��������к͵ζ�����������������Ŀ�Ѷ��еȣ�ע�������к͵ζ��IJ����������ζ��յ���жϷ������ζ����ķ��������뼼�ɣ���������������ѧ�����Ӧ����ѧ֪ʶ���ʵ�������������

 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�| A�� | 0.2mol/��L•S�� | B�� | 0.1mol/��L•S�� | C�� | 0.04mol/��L•S�� | D�� | 0.08mol/��L•S�� |
| A�� | �⻯�ﲻ�ȶ� | |
| B�� | ����������Ӧ��ˮ������һ��ǿ�� | |
| C�� | �䵥�ʼ������������л�ԭ�� | |
| D�� | ��ֲ����������Ҫ��һ��Ӫ��Ԫ�� |
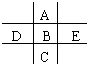 ��ͼΪ���ڱ���һС���֣�A��B��C��D��E��λ�ù�ϵ��ͼ��ʾ������BԪ�ص��������������۾���ֵ��3������������������к���60%���ش��������⣺
��ͼΪ���ڱ���һС���֣�A��B��C��D��E��λ�ù�ϵ��ͼ��ʾ������BԪ�ص��������������۾���ֵ��3������������������к���60%���ش��������⣺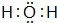 ��
�� ��
�� ��ͼΪ�Զ��Ե缫���е�⣺
��ͼΪ�Զ��Ե缫���е�⣺ �����һ��ʵ�飬��֤���ȵ�̿��Ũ���ᷢ����Ӧ�����ɵĸ��ֲ����һλͬѧ��������������������һ��ʵ�����̣�����ʹ�õ��Լ�˳��ɱ�ʾΪ������������ˮ����ͭ�����Ʒ����Һ�����Ը��������Һ��Ʒ����Һ������ʯ��ˮ��
�����һ��ʵ�飬��֤���ȵ�̿��Ũ���ᷢ����Ӧ�����ɵĸ��ֲ����һλͬѧ��������������������һ��ʵ�����̣�����ʹ�õ��Լ�˳��ɱ�ʾΪ������������ˮ����ͭ�����Ʒ����Һ�����Ը��������Һ��Ʒ����Һ������ʯ��ˮ��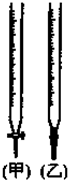 ���к͵ζ����ⶨ�ռ�Ĵ��ȣ����ռ��к��������Ӧ�����ʣ��Ը���ʵ��ش�
���к͵ζ����ⶨ�ռ�Ĵ��ȣ����ռ��к��������Ӧ�����ʣ��Ը���ʵ��ش�
