��Ŀ����
10������һ������ɫ��������ѧ�����ȶ�����+2��+3��+6��Ϊ������̬����ҵ���Ը�������Ҫ�ɷ�ΪFeO•Cr2O3������Al2O3��SiO2�����ʣ�Ϊ��Ҫԭ���������������ظ�����Na2Cr2O7•2H2O����֪Na2Cr2O7��һ��ǿ��������������Ҫ����������ͼ1���������ϵ�֪���ٳ����£�NaBiO3������ˮ����ǿ�����ԣ��ڼ��������£��ܽ�Cr3+ת��ΪCrO42-
�ڳ����£�Ksp[Cr��OH��3]=6.3��10-31
�ش��������⣺
��1����ҵ�ϳ������Ȼ�ԭ���Ʊ���������д����Cr2O3Ϊԭ�ϣ��������ȷ�Ӧ��ȡ�������Ļ�ѧ����ʽCr2O3+2Al$\frac{\underline{\;����\;}}{\;}$2Cr+Al2O3��
��2���ữ��ҺDʱ����ѡ�������ԭ���������е�Cl-�ᱻ����������Cl2��
��3������E����Ҫ�ɷ���Na2SO4������ͼ2��������aΪ�����ᾧ�����ȹ��ˣ�
��4����֪��+6�۸�����ˮ����Ⱦ��������Ƴ������Ķ�ͭ��ˮ����������һ������Cr2O72-�������÷�ˮ���û�ԭ������������������ͼ3��

��Cr��OH��3�Ļ�ѧ������Al��OH��3���ƣ����������������м���NaOH��ҺʱҪ������Һ��pH���ܹ��ߣ�����ΪpH����Cr��OH��3���������NaOH��Ӧ��
��������Һ�п��Դ�������������Na2S2O3��Һ����D����ѡ����ţ���
A��FeSO4��Һ B��ŨH2SO4 C������KMnO4��ҺD��Na2SO3��Һ
�۵�����Һ��pH=5ʱ��ͨ����ʽ����˵����Һ�е�Cr3+�Ƿ������ȫc��Cr3+��•��10-9��3=6.3��10-31����֮��c��Cr3+��=6.3��10-4��10-5������û�г�����ȫ��
�����������У�ÿ����0.1molNa2S2O3ת��0.8mole-�������Na2S2O3��Һʱ������Ӧ�����ӷ���ʽΪ3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O��
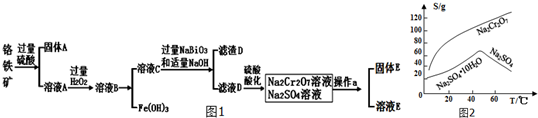
���� ��������Ҫ�ɷ�ΪFeO��Cr2O3������Al2O3��SiO2�����ʣ����������ϡ���ᣬ����AΪSiO2����ҺA�к���Cr3+��Al3+��Fe2+����A�м���������⣬������Fe3+��������ҺpH�ɳ�ȥFe3+��Al3+������������������������������������D����ҺC����Cr3+������ҺC�м���NaBiO3��NaOH������������ԭ��Ӧ������DΪBi��OH��3����ҺD����Na2CrO4���ữ�ɵ�Na2Cr2O7����Һ������Ũ������ȴ�ᾧ�ɵ�Na2Cr2O7•2H2O���Դ˽����⣮
��1�����������������ڸ��������·����û���Ӧ���ɸ���������������
��2�������е�����-1�۾��л�ԭ�ԣ���CrO42-��������������
��3����ͼ2��֪Na2Cr2O7���ܽ�����¶ȵ����߶����������Ƶ��¶����¶ȵ����߶����ͣ����Բ��������ᾧ�����ȹ��˵ķ������������ƣ�
��4����Cr��OH��3�Ļ�ѧ������Al��OH��3���ƣ�����������ƹ���Cr��OH��3���ܽ⣻
�ڿ��Դ�������������Na2S2O3��Һ����Ҫ���л�ԭ�ԣ��ܻ�ԭ�ظ�������ӣ�
�۸���Ksp[Cr��OH��3]=c��Cr3+��•��OH-��3������������ӵ�Ũ���Ƿ�С��10-5mol/L��
��ÿ����0.1mol Na2S2O3ת��0.8mol e-��Na2S2O3 ��2SO42-��8e-��Cr2O72-��2Cr3+��6e-������������ԭ��Ӧ�����غ������ƽ��д������ԭ��Ӧ�����ӷ���ʽ��
��� �⣺��1�����������������������ȷ�Ӧ�ķ���ʽΪ��Cr2O3+2Al$\frac{\underline{\;����\;}}{\;}$2Cr+Al2O3���ʴ�Ϊ��Cr2O3+2Al$\frac{\underline{\;����\;}}{\;}$2Cr+Al2O3 ��
��2�������е�����-1�۾��л�ԭ�ԣ���CrO42-���������������ʴ�Ϊ�������е�Cl-�ᱻ����������Cl2��
��3����ͼ2��֪Na2Cr2O7���ܽ�����¶ȵ����߶����������Ƶ��¶����¶ȵ����߶����ͣ����Բ��������ᾧ�����ȹ��˵ķ������������ƣ��ʴ�Ϊ�������ᾧ�����ȹ��ˣ�
��4����Cr��OH��3�Ļ�ѧ������Al��OH��3���ƣ�����������ƹ���Cr��OH��3���ܽ⣬�ʴ�Ϊ��pH����Cr��OH��3���������NaOH ��Ӧ��
�ڿ��Դ�������������Na2S2O3��Һ����Ҫ���л�ԭ�ԣ��ܻ�ԭ�ظ�������ӣ�
A��FeSO4��Һ���������Ӿ��л�ԭ�ԣ����Ի�ԭCr2O72-���ӣ����������µ��������������ӣ���A�����ϣ�
B��ŨH2SO4 ����ǿ�����ԣ����ܱ��ֻ�ԭ�ԣ����ܻ�ԭCr2O72-����B�����ϣ�
C������KMnO4 ��ǿ���������ܻ�ԭCr2O72-����C�����ϣ�
D��Na2SO3��Һ������������Ӿ��л�ԭ�ԣ����Ի�ԭCr2O72-����D���ϣ�
�ʴ�Ϊ��D��
��pH=5ʱ����OH-��=10-9mol/L������c��Cr3+��•��10-9��3=6.3��10-31����֮��c��Cr3+��=6.3��10-4��10-5������û�г�����ȫ���ʴ�Ϊ��c��Cr3+��•��10-9��3=6.3��10-31����֮��c��Cr3+��=6.3��10-4��10-5������û�г�����ȫ��
��ÿ����0.1mol Na2S2O3ת��0.8mol e-��Na2S2O3 ��2SO42-��8e-��Cr2O72-��2Cr3+��6e-������������ԭ��Ӧ�����غ���ƽ��д��3Na2S2O3 ��6SO42-��24e-��4Cr2O72-��8Cr3+��24e-���õ���������ԭ��Ӧ�����ӷ���ʽΪ3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O��
�ʴ�Ϊ��3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O��
���� ���⿼�������ʷ����ᴿ�ķ������̷��������ӷ���ʽ��д���ܶȻ������ļ���Ӧ�ã���Ҫ��������ԭ��Ӧ������Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д�| A�� | ��������Һ�������Һ��ϣ�SiO32-+2H+=H2SiO3�� | |
| B�� | NH4Al��SO4��2��Һ�����ϡ��ˮ��Ӧ��Al3++3NH3•H2O=Al��OH��3��+3NH4+ | |
| C�� | ��ϡ������ϴ�Թ��ڱڵ�������Ag+2H++NO3-=Ag++NO2��+H2O | |
| D�� | FeBr2��Һ��ͨ�����Cl2��2Fe2++2Br-+2Cl2=2Fe3++Br2+4Cl- |
| A�� | Y��������Y 2-�Ļ�ԭ��ǿ��X��������X- | |
| B�� | X�ĺ���������Ա�Y�ĺ����������ǿ | |
| C�� | X�ĵ���X2�ܽ�Y��������Y 2-�������������û���Ӧ | |
| D�� | X���⻯���Y���⻯���ȶ� |
| A�� | 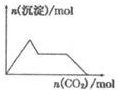 ��0.0l mol KOH��0.01 mol Ca��OH��2�Ļ����Һ�л���ͨ��CO2 | |
| B�� | 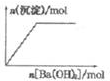 NaHSO4��Һ����μ���Ba��OH��2��Һ | |
| C�� | 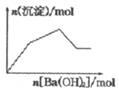 KAl��SO4��2��Һ����μ���Ba��OH��2��Һ | |
| D�� | 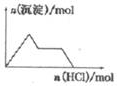 NaAlO2��Һ����μ������� |
| A�� | pH=2��HA��Һ��pH=12��MOH��Һ������Ȼ�ϣ�c��H+��+c��M+��=c��OH-��+c��A-�� | |
| B�� | pH=10��Na2A��Һ�У�2 c��Na+��=c��HA-��+c��H2A��+c��A2-�� | |
| C�� | �����ʵ���Ũ�ȵ�NaClO��NaHCO3�����Һ�У�c��HClO��+c��ClO-��=c��HCO3-��+c��H2CO3��+c��CO32-�� | |
| D�� | ��ˮ���Ȼ�淋Ļ����Һ�����ܻ����c��NH${\;}_{4}^{+}$����c��Cl-����c��H+����c��OH-�� |
| A�� | ������ | B�� | ������̼��ɸɱ� | ||
| C�� | �Ȼ�����������ˮ | D�� | �����ھƾ� |
| A�� | 0.54g | B�� | 1.08g | C�� | 1.62g | D�� | 2.16g |
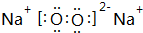 ��д��M2Z2��ˮ��Ӧ�����ӷ���ʽ��2Na2O2+2H2O=4Na++4OH-+O2����
��д��M2Z2��ˮ��Ӧ�����ӷ���ʽ��2Na2O2+2H2O=4Na++4OH-+O2���� ����������Դ�������Ҫ��Ա������������������ص����⣮
����������Դ�������Ҫ��Ա������������������ص����⣮