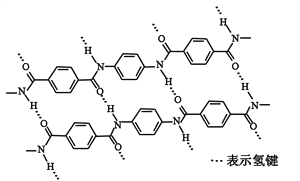
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ»ΐ¬»Μ·ΝυΑ±Κœνή[Co(NH3)6]Cl3 «≥»ΜΤ…ΪΓΔΈΔ»ή”ΎΥ°ΒΡ≈δΚœΈοΘ§ «Κœ≥…ΤδΥϋ“Μ–©Κ§νή≈δΚœΈοΒΡ‘≠ΝœΓΘœ¬ΆΦ «Ρ≥ΩΤ―––ΓΉι“‘Κ§νήΖœΝœΘ®Κ§…ΌΝΩFeΓΔAl Β»‘”÷ Θ©÷Τ»Γ[Co(NH3)6]Cl3 ΒΡΙΛ“’Νς≥ΧΘΚ
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©–¥≥ωΦ”ΓΑ ΝΩNaClO3Γ±ΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ______________ΓΘ
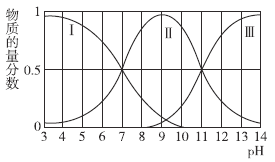
Θ®2Θ©ΓΑΦ”Na2CO3 ΒςpH÷ΝaΓ±Μα…ζ≥…ΝΫ÷÷≥ΝΒμΘ§Ζ÷±πΈΣ_______________________Θ®ΧνΜ·―ß ΫΘ©ΓΘ
Θ®3Θ©≤ΌΉςΔώΒΡ≤Ϋ÷ηΑϋά®_____________________________ΓΔά以ΫαΨßΓΔΦθ―ΙΙΐ¬ΥΓΘ
Θ®4Θ©Νς≥Χ÷–NH4Cl≥ΐΉςΖ¥”ΠΈοΆβΘ§ΜΙΩ…Ζά÷ΙΦ”Α±Υ° ±c(OHΘ≠) Ιΐ¥σΘ§Τδ‘≠άμ «_________________ΓΘ
Θ®5Θ©ΓΑ―θΜ·Γ±≤Ϋ÷ηΘ§ΦΉΆ§―ß»œΈΣ”Πœ»Φ”»κΑ±Υ°‘ΌΦ”»κH2O2Θ§““Ά§―ß»œΈΣ ‘ΦΝΧμΦ”Υ≥–ρΕ‘≤ζΈοΈό”ΑœλΓΘΡψ»œΈΣ___________Θ®ΧνΓΑΦΉΓ±ΜρΓΑ““Γ±Θ©Ά§―ßΙέΒψ’ΐ»ΖΘ§άμ”… «_________________________________ΓΘ–¥≥ωΗΟ≤Ϋ÷ηΒΡΜ·―ßΖΫ≥Χ ΫΘΚ________________________________
Θ®6Θ©Ά®ΙΐΒβΝΩΖ®Ω…≤βΕ®≤ζΤΖ÷–νήΒΡΚ§ΝΩΓΘΫΪ [Co(NH3)6]Cl3 ΉΣΜ·≥…Co3ΘΪΚσΘ§Φ”»κΙΐΝΩKI »ή“ΚΘ§‘Ό”ΟNa2S2O3±ξΉΦ“ΚΒΈΕ®(ΒμΖέ»ή“ΚΉω÷Η ΨΦΝ)Θ§Ζ¥”Π‘≠άμΘΚ2Co3ΘΪΘΪ2IΘ≠ΘΫ2Co2ΘΪΘΪI2Θ§I2ΘΪ2S2O32Θ≠ΘΫ2IΘ≠ΘΪS4O62Θ≠Θ§ Β―ιΙΐ≥Χ÷–Θ§œ¬Ν–≤ΌΉςΜαΒΦ÷¬Υυ≤βνήΚ§ΝΩ ΐ÷ΒΤΪΗΏΒΡ «_______ΓΘ
aΘ°”ΟΨΟ÷Ο”ΎΩ’Τχ÷–ΒΡ KI ΙΧΧε≈δ÷Τ»ή“Κ
bΘ° ΔΉΑNa2S2O3±ξΉΦ“ΚΒΡΦν ΫΒΈΕ®ΙήΈ¥»σœ¥
cΘ°ΒΈΕ®Ϋα χΚσΘ§ΖΔœ÷ΒΈΕ®ΙήΡΎ”–Τχ≈ί
dΘ°»ή“ΚάΕ…ΪΆΥ»ΞΘ§ΝΔΦ¥ΕΝ ΐ
ΓΨ¥πΑΗΓΩ6Fe2ΘΪΘΪClO3Θ≠ΘΪ6HΘΪ=6Fe3ΘΪΘΪClΘ≠ΘΪ3H2O Fe(OH)3 ΚΆ Al(OH)3 HClΖ’Έßœ¬’τΖΔ≈®Υθ NH4Cl»ή”ΎΥ°Βγάκ≥ωNH4ΘΪΜα“÷÷ΤΚσΤΎΦ”»κΒΡNH3ΓΛH2OΒΡΒγάκ ΦΉ Ζά÷ΙCo(OH)3ΒΡ…ζ≥… H2O2ΘΪ2CoCl2ΘΪ2NH4ClΘΪ10NH3ΓΛH2OΘΫ2Co(NH3)6Cl3ΓΐΘΪ12H2O ab
ΓΨΫβΈωΓΩ
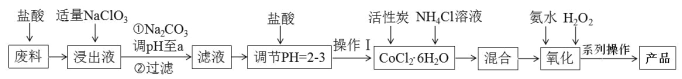
“‘Κ§νήΖœΝœΘ®Κ§…ΌΝΩFeΓΔAl Β»‘”÷ Θ©÷Τ»Γ[Co(NH3)6]Cl3 Θ§”Ο―ΈΥα»ήΫβΖœΝœΘ§ΒΟΒΫCo2+ΓΔFe2+ΓΔAl3+ΒΡΥα–‘»ή“ΚΘ§Φ”»κ ΝΩΒΡNaClO3ΫΪ―«ΧζάκΉ”―θΜ·ΈΣΧζάκΉ”Θ§‘ΌΦ”»κΧΦΥαΡΤΒςΫΎpHΘ§≥ΝΒμ¬ΝάκΉ”ΓΔΧζάκΉ”ΈΣ«β―θΜ·¬ΝΚΆ«β―θΜ·ΧζΘ§Ιΐ¬ΥΘ§ΒΟΒΫ¬Υ“ΚΘ§œρΚ§”–Co2+ΒΡ»ή“Κ÷–Φ”»κ―ΈΥαΒςΫΎpH=2-3,Φ”»κΜν–‘ΧΩΚΆ¬»Μ·οß»ή“ΚΒΟΒΫCoCl2 6H2OΘ§‘Ό“ά¥ΈΦ”»κΑ±Υ°ΚΆΙΐ―θΜ·«βΘ§ΖΔ…ζΖ¥”ΠH2O2ΘΪ2CoCl2ΘΪ2NH4ClΘΪ10NH3ΓΛH2OΘΫ2Co(NH3)6Cl3ΓΐΘΪ12H2OΘ§‘ΌΫΪ≥ΝΒμ‘Ύ¬»Μ·«βΖ÷ΈΣœ¬’τΖΔ≈®ΥθΘ§ά以ΫαΨßΘ§Φθ―ΙΙΐ¬ΥΒΟΒΫ≤ζΤΖΘ§Ψί¥ΥΖ÷ΈωΉς¥πΓΘ
(1)Φ” ΝΩNaClO3ΒΡΡΩΒΡ «ΫΪ―«ΧζάκΉ”―θΜ·ΈΣΧζάκΉ”Θ§ΖΔ…ζΒΡάκΉ”Ζ¥”ΠΈΣ6Fe2ΘΪΘΪClO3Θ≠ΘΪ6HΘΪ=6Fe3ΘΪΘΪClΘ≠ΘΪ3H2OΘΜ
(2)Φ”»κΧΦΥαΡΤΒςΫΎpHΘ§≥ΝΒμ¬ΝάκΉ”ΚΆΧζάκΉ”Θ§ΉΣΜ·ΈΣFe(OH)3 ΚΆ Al(OH)3ΘΜ
(3)ΈΔΝΘΖά÷Ι≤ζΤΖΥ°ΫβΘ§Ι ”Π‘ΎHClΖ’Έßœ¬’τΖΔ≈®ΥθΘΜ
(4)Νς≥Χ÷–¬»Μ·οß≥ΐΉςΈΣΖ¥”ΠΈοΆβΘ§NH4Cl»ή”ΎΥ°Βγάκ≥ωNH4ΘΪΜα“÷÷ΤΚσΤΎΦ”»κΒΡNH3ΓΛH2OΒΡΒγάκΘ§Ω…Ζά÷ΙΦ”Α±Υ° ±«β―θΗυάκΉ”≈®Ε»Ιΐ¥σΘΜ
(5)»τœ»Φ”»κΙΐ―θΜ·«βΘ§ΫΪνή‘ΣΥΊ―θΜ·ΒΫCo3+Θ§ΚσΦ”»κΑ±Υ°Θ§Μα…ζ≥…«β―θΜ·νήΘ§≤Μάϊ”Ύ≤ζΤΖΒΡ…ζ≥…Θ§Ι ΦΉΆ§―ß’ΐ»ΖΘ§œ»Φ”»κΑ±Υ°‘ΌΦ”»κΙΐ―θΜ·«βΘ§Ω…Ζά÷ΙCo(OH)3ΒΡ…ζ≥…Θ§¥Υ ±ΒΡΖ¥”ΠΈΣH2O2ΘΪ2CoCl2ΘΪ2NH4ClΘΪ10NH3ΓΛH2OΘΫ2Co(NH3)6Cl3ΓΐΘΪ12H2OΘΜ
(6) aΘ°”ΟΨΟ÷Ο”ΎΩ’Τχ÷–ΒΡ KI ΙΧΧε≈δ÷Τ»ή“ΚΘ§ΒβΜ·ΦΊ≤ΩΖ÷±Μ―θΜ·ΈΣΒβΒΞ÷ Θ§ΒΈΕ® ±œϊΚΡΒΡΝρ¥ζΝρΥαΡΤ±ξΉΦ‘ωΕύΘ§≤βΕ®ΫαΙϊΤΪΗΏΘ§Ι ’ΐ»ΖΘΜ
bΘ° ΔΉΑNa2S2O3±ξΉΦ“ΚΒΡΦν ΫΒΈΕ®ΙήΈ¥»σœ¥Θ§‘ρ±ξΉΦ“≤±ΜœΓ ΆΘ§ΒΈΕ® ±œϊΚΡΒΡΝρ¥ζΝρΥαΡΤ±ξΉΦ“≤ΧεΜΐ‘ωΕύΘ§≤βΕ®ΫαΙϊΤΪΗΏΘ§Ι ’ΐ»ΖΘΜ
cΘ°ΒΈΕ®Ϋα χΚσΘ§ΖΔœ÷ΒΈΕ®ΙήΡΎ”–Τχ≈ίΘ§Τχ≈ί’ΦΧεΜΐΘ§‘ρœϊΚΡΒΡΝρ¥ζΝρΥαΡΤ±ξΉΦ“ΚΧεΜΐΕΝ ΐΤΪ–ΓΘ§≤βΕ®ΫαΙϊΤΪΒΆΘ§Ι ¥μΈσΘΜ
dΘ°»ή“ΚάΕ…ΪΆΥ»ΞΘ§ΝΔΦ¥ΕΝ ΐΘ§¥Υ ±»ή“ΚΜλΚœ≤ΜΨυΘ§ΒβΒΞ÷ ≤ΔΈ¥Άξ»Ϊ±Μ±ξΉΦ“ΚΖ¥”ΠΆξ»ΪΘ§ΒΦ÷¬œϊΚΡΒΡ±ξΉΦ“ΚΦθ…ΌΘ§≤βΕ®ΫαΙϊΤΪΒΆΘ§Ι ¥μΈσΓΘΙ ―ΓabΓΘ

 ΦΤΥψΗΏ ÷œΒΝ–¥πΑΗ
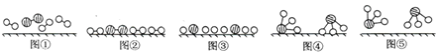
ΦΤΥψΗΏ ÷œΒΝ–¥πΑΗΓΨΧβΡΩΓΩ Β―ι “Ω…άϊ”ΟΜΖΦΚ¥ΦΒΡ―θΜ·Ζ¥”Π÷Τ±ΗΜΖΦΚΆΣΘ§Ζ¥”Π‘≠άμΚΆ Β―ιΉΑ÷Ο(≤ΩΖ÷Φ–≥÷ΉΑ÷Ο¬‘)»γΆΦΘΚ
![]()
![]()
![]()
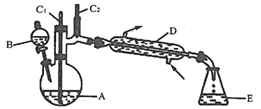
”–ΙΊΈο÷ ΒΡΈοάμ–‘÷ Φϊ±μΓΘ
Έο÷ | Ζ–Βψ(Γφ) | ΟήΕ»(gΓΛcm3Θ§20Γφ) | »ήΫβ–‘ |
ΜΖΦΚ¥Φ | 161.1(97.8)* | 0.96 | Ρή»ή”ΎΥ°ΚΆΟ― |
ΜΖΦΚΆΣ | 155.6(95.0)* | 0.95 | ΈΔ»ή”ΎΥ°Θ§Ρή»ή”ΎΟ― |
Υ° | 100.0 | 1.0 |
*ά®Κ≈÷–ΒΡ ΐΨί±μ ΨΗΟ”–ΜζΈο”κΥ°–Έ≥…ΒΡΨΏ”–ΙΧΕ®Ήι≥…ΒΡΜλΚœΈοΒΡΖ–ΒψΓΘ
Β―ιΆ®ΙΐΉΑ÷ΟBΫΪΥα–‘Na2Cr2O7»ή“ΚΦ”ΒΫ Δ”–10mLΜΖΦΚ¥ΦΒΡA÷–Θ§55ΓΪ60ΓφΫχ––Ζ¥”ΠΓΘΖ¥”ΠΆξ≥…ΚσΘ§Φ”»κ ΝΩΥ°Θ§’τΝσΘ§ ’Φ·95ΓΪ100ΓφΒΡΝσΖ÷Θ§ΒΟΒΫ÷ς“ΣΚ§ΜΖΦΚΆΣ¥÷ΤΖΚΆΥ°ΒΡΜλΚœΈοΓΘ
(1)ΉΑ÷ΟDΒΡΟϊ≥ΤΈΣ___________ΓΘ
(2)Υα–‘Na2Cr2O7»ή“Κ―θΜ·ΜΖΦΚ¥ΦΖ¥”ΠΒΡHΘΦ0Θ§Ζ¥”ΠΨγΝ“ΫΪΒΦ÷¬ΧεœΒΈ¬Ε»―ΗΥΌ…œ…ΐΘ§Η±Ζ¥”Π‘ωΕύΓΘ
ΔΌΒΈΦ”Υα–‘Na2Cr2O7»ή“Κ ±Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ__________ΘΜ
ΔΎ’τΝσ≤ΜΡήΖ÷άκΜΖΦΚΆΣΚΆΥ°ΒΡ‘≠“ρ «____________ΓΘ
(3)ΜΖΦΚΆΣΒΡΧα¥Ω–η“ΣΨ≠Ιΐ“‘œ¬“ΜœΒΝ–ΒΡ≤ΌΉςΘΚ
a.’τΝσΓΔ≥ΐ»Ξ““Ο―ΚσΘ§ ’Φ·151ΓΪ156ΓφΝσΖ÷
b.Υ°≤ψ”Ο““Ο―(““Ο―Ζ–Βψ34.6ΓφΘ§“Ή»Φ…’)ίΆ»ΓΘ§ίΆ»Γ“Κ≤Δ»κ”–Μζ≤ψ
c.Ιΐ¬Υ
d.Άυ“ΚΧε÷–Φ”»κNaClΙΧΧε÷Ν±ΞΚΆΘ§Ψ≤÷ΟΘ§Ζ÷“Κ
e.Φ”»κΈόΥ°MgSO4ΙΧΧεΘ§≥ΐ»Ξ”–ΜζΈο÷–…ΌΝΩΒΡΥ°
ΔΌ…œ ωΧα¥Ω≤Ϋ÷ηΒΡ’ΐ»ΖΥ≥–ρ «_____________ΘΜ
ΔΎb÷–Υ°≤ψ”Ο““Ο―ίΆ»ΓΒΡΡΩΒΡ «___________ΘΜ
Δέ…œ ω≤ΌΉςcΓΔd÷– Ι”ΟΒΡ≤ΘΝß“«Τς≥ΐ…’±≠ΓΔΉΕ–ΈΤΩΓΔ≤ΘΝßΑτΆβΘ§ΜΙ–η“ΣΒΡ≤ΘΝß“«Τς”–_______Θ§≤ΌΉςd÷–Θ§Φ”»κNaClΙΧΧεΒΡΉς”Ο «____________ΓΘ
(4)Μ÷Η¥÷Ν “Έ¬ ±Θ§Ζ÷άκΒΟΒΫ¥Ω≤ζΤΖΧεΜΐΈΣ6mLΘ§‘ρΜΖ“―ΆΣΒΡ≤ζ¬ ____________ΓΘ(ΦΤΥψΫαΙϊΨΪ»ΖΒΫ0.1%)