��Ŀ����
����Ŀ��ú���еĵ�Ԫ����ʹ�ù����е�ת����ϵ��ͼ��ʾ��

(1)����NH3���뷴Ӧ�Ļ�ѧ����ʽΪ_______��
(2)��̿������һ�ֳ����ĺ����л������(![]() )������������ڵ�C��Nԭ����ȣ�Nԭ����������������___________(����ǿ����������)����ԭ�ӽṹ�ǶȽ���ԭ��________��
)������������ڵ�C��Nԭ����ȣ�Nԭ����������������___________(����ǿ����������)����ԭ�ӽṹ�ǶȽ���ԭ��________��
(3)��ҵ�ϳɰ����˹��̵�����Ҫ������2007�껯ѧ�Ҹ���������ض�֤ʵ�������뵪���ڹ����������ϳɰ��ķ�Ӧ���̣�ʾ����ͼ��

����˵����ȷ����________(ѡ����ĸ)��
a. ͼ�ٱ�ʾN2��H2�����о��ǵ���
b. ͼ����ͼ����Ҫ��������
c. �ù��̱�ʾ�˻�ѧ�仯�а����ɻ�ѧ���Ķ��Ѻ��»�ѧ��������
(4)��֪��N2(g) �� O2(g) = 2NO(g) ��H = a kJ��mol-1
N2(g) �� 3H2(g) = 2NH3(g) ��H = b kJ��mol-1
2H2(g) �� O2(g) = 2H2O(l) ��H = c kJ��mol-1
��Ӧ��ָ������³�ѹ������NH3���뷴Ӧ���Ȼ�ѧ����ʽΪ________��
(5)�ü�ӵ绯ѧ����ȥNO�Ĺ��̣���ͼ��ʾ��

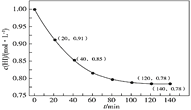
����֪���ص�����������Һ��pH��4~7֮�䣬д�������ĵ缫��Ӧʽ��________��
�������ӷ���ʽ��ʾ���ճ��г�ȥNO��ԭ����__________��
���𰸡�4NH3+5O2 4NO+6H2O ǿ C��Nԭ����ͬһ����(����Ӳ�����ͬ)��Nԭ�Ӻ˵��������ԭ�Ӱ뾶��С��ԭ�Ӻ˶������ӵ���������ǿ bc 4NH3(g) + 6NO(g) = 5N2(g) + 6H2O(l) ��H = (3c-3a-2b) kJ��mol-1 2HSO3- + 2e- + 2H+ = S2O42- + 2H2O 2NO + 2S2O42- +2H2O = N2 + 4HSO3-
4NO+6H2O ǿ C��Nԭ����ͬһ����(����Ӳ�����ͬ)��Nԭ�Ӻ˵��������ԭ�Ӱ뾶��С��ԭ�Ӻ˶������ӵ���������ǿ bc 4NH3(g) + 6NO(g) = 5N2(g) + 6H2O(l) ��H = (3c-3a-2b) kJ��mol-1 2HSO3- + 2e- + 2H+ = S2O42- + 2H2O 2NO + 2S2O42- +2H2O = N2 + 4HSO3-
��������
(1)�����ڴ�����������������Ӧ����һ��������ˮ��Ϊ��Ҫ�Ĺ�ҵ��Ӧ����Ӧ�Ļ�ѧ����ʽΪ4NH3+5O2 4NO+6H2O��
4NO+6H2O��
(2)����C��Nԭ����ͬһ����(����Ӳ�����ͬ)��Nԭ�Ӻ˵��������ԭ�Ӱ뾶��С��ԭ�Ӻ˶������ӵ���������ǿ������Nԭ����������������ǿ��
(3)a��������������ԭ��֮��Ϊ��������a����
b����������ͼ����֪����ͼ�ڱ�ʾN2��H2�������ڴ������棬��ͼ�۱�ʾ�ڴ������棬N2��H2�л�ѧ�����ѣ��ϼ���������������ͼ����ͼ����Ҫ������������b��ȷ��
c���ڻ�ѧ�仯�У������Ӻ�������ڴ����������¶��ѳ���ԭ�Ӻ͵�ԭ�ӣ�������ѧ���Ķ��ѣ�Ȼ��ԭ����������ϳ��µķ��ӣ��γ��µĻ�ѧ�������Ըù��̱�ʾ�˻�ѧ�仯�а����ɻ�ѧ���Ķ��Ѻ��»�ѧ�������ɣ���c��ȷ��
��ѡbc��
(4)����NH3����ķ�ӦΪ��4NH3(g) + 6NO(g) = 5N2(g) + 6H2O(l)��
��֪��N2(g) �� O2(g) = 2NO(g) ��H = a kJ��mol-1 i��
N2(g) �� 3H2(g) = 2NH3(g) ��H = b kJ��mol-1 ii��
2H2(g) �� O2(g) = 2H2O(l) ��H = c kJ��mol-1 iii��
���ݸ�˹����iii��3- i��3-ii��2�ɵ�4NH3(g) + 6NO(g) = 5N2(g) + 6H2O(l) ��H=(3c-3a-2b)kJ��mol-1��
(5)������������ԭ��Ӧ����ͼ��֪������������ӵõ��ӱ���ԭ����S2O42-���������Һ�������ԣ����Ե缫��ӦʽΪ��2HSO3-+2e-+2H+=S2O42-+2H2O��
�ھ�ͼ��֪S2O42-��һ����������������ԭ��Ӧ�����ɵ�������������������ݵ�ʧ�����غ㡢ԭ���غ�͵���غ㣬��Ӧ�����ӷ���ʽΪ��2NO+2S2O42-+2H2O=N2+4HSO3-��

����Ŀ��һ���¶�ʱ����2.0 L�����ܱ������г���2 mol SO2��1 mol O2��������Ӧ��2SO2(g)��O2(g)![]() 2SO3(g)������һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
2SO3(g)������һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
t / s | 0 | 2 | 4 | 6 | 8 |
n(SO3) / mol | 0 | 0��8 | 1��4 | 1.8 | 1.8 |
����˵����ȷ����( )
A. ��Ӧ��ǰ2 s ��ƽ������v(O2) �� 0��4 mol��L��1��s��1
B. ���������������䣬���ѹ����1.0 L��ƽ�ⳣ��������
C. ��ͬ�¶��£���ʼʱ�������г���4 mol SO3���ﵽƽ��ʱ��SO3��ת����С��10%
D. �����¶Ȳ��䣬����������ٳ���2 mol SO2��1 mol O2����Ӧ�ﵽ��ƽ��ʱn(SO3)/n(O2)��С
����Ŀ��������ҩƷ��ǩ���йط�������ȷ���ǣ��� ����
ѡ�� | A | B | C | D |
��Ʒ��ǩ | ������ˮ1.01��105 Pa��20 �� | ҩƷ��������
| ̼������NaHCO3����С�մ�84 g��mol��1�� | Ũ����H2SO4 �ܶ�1.84 g��mL��1Ũ��98.0% |
���� | ���Լ�Ӧװ��������ϸ��ƿ�� | ��ҩƷ������Ƥ��ֱ�ӽӴ� | �����������ֽ� | ��ҩƷ��ǩ�ϻ�����
|
A. AB. BC. CD. D