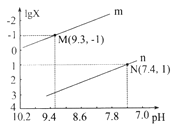
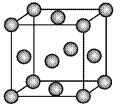
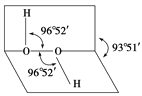
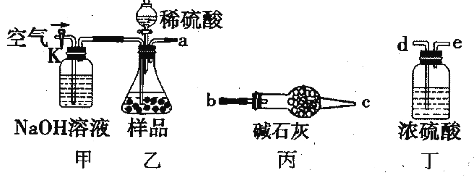
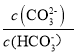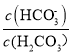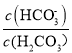��Ŀ����
����Ŀ���жϺ���������ǿ����һ����������ǣ���������ӽṹ�к����ǻ���ԭ����Խ�࣬�ú����������Խǿ�����±���ʾ��
����������ǿ������ǻ���ԭ�����Ĺ�ϵ
������ | ���� | ���� | ������ | |
������ | Cl��OH |
|
|
|
���ǻ���ԭ���� | 0 | 1 | 2 | 3 |
���� | ���� | ��ǿ�� | ǿ�� | ��ǿ�� |
(1)������H3PO3��������H3AsO3����ʽ���ƣ������ǵ�����ǿ�����ܴ�H3PO3����ǿ�ᣬH3AsO3�������������������ԡ��ɴ˿�֪���ǵĽṹʽ�ֱ�Ϊ��__________����__________��
(2)H3PO3��H3AsO3�������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ�ֱ��Ǣ�__________����__________��
(3)��H3PO3��H3AsO3�зֱ����Ũ���ᣬ������Ӧ���__________��д����Ӧ�Ļ�ѧ����ʽ________________________________________________________________________��
���𰸡� 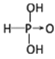
![]() H3PO3+2NaOH=Na2HPO3+2H2O H3AsO3+3NaOH=Na3AsO3+3H2O ������������Ӧ��������������ܷ�Ӧ����AsCl3��ˮ As��OH��3+3HCl=AsCl3+3H2O
H3PO3+2NaOH=Na2HPO3+2H2O H3AsO3+3NaOH=Na3AsO3+3H2O ������������Ӧ��������������ܷ�Ӧ����AsCl3��ˮ As��OH��3+3HCl=AsCl3+3H2O
����������1����������ӽṹ�к����ǻ���ԭ����Խ�࣬�ú����������Խǿ������������ǿ�ᣬ������������������������ԣ������������������Ľṹʽ�ֱ�Ϊ����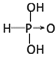 ����
����![]() ����2����ͼӦ�����κ�ˮ���������к���2���ǻ������ڶ�Ԫ�ᣬ������������Ԫ�ᣬ�������������ֱ���������Ƶķ�Ӧ����ʽΪ����H3PO3+2NaOH=Na2HPO3+2H2O����H3AsO3+3NaOH=Na3AsO3+3H2O����3������������ǿ�ᣬ������������������������ԣ�����������������Ӧ��������������ܷ�Ӧ����AsCl3��ˮ����Ӧ����ʽΪAs��OH��3+3HCl=AsCl3+3H2O��
����2����ͼӦ�����κ�ˮ���������к���2���ǻ������ڶ�Ԫ�ᣬ������������Ԫ�ᣬ�������������ֱ���������Ƶķ�Ӧ����ʽΪ����H3PO3+2NaOH=Na2HPO3+2H2O����H3AsO3+3NaOH=Na3AsO3+3H2O����3������������ǿ�ᣬ������������������������ԣ�����������������Ӧ��������������ܷ�Ӧ����AsCl3��ˮ����Ӧ����ʽΪAs��OH��3+3HCl=AsCl3+3H2O��

 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д�