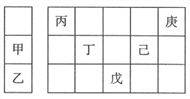
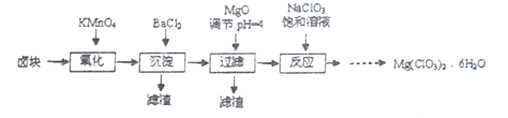
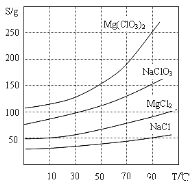
��Ŀ����
����Ŀ����֪AΪʯ���ѽ����Ҫ����,��Է�������Ϊ28��BΪ����,�ڱ�״���µ��ܶ�Ϊ1.965g��L-1��D��F��Ϊͬ���칹��,F�ǻ�״�����G�Ľṹ��ʽΪ��![]() ����һ�������£���������ͼ��ʾ��ת����ϵ��
����һ�������£���������ͼ��ʾ��ת����ϵ��
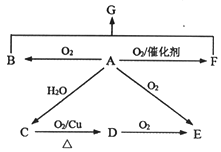
��ش���
��1��E����������������________��
��2��B��F��Ӧ����G�ķ�Ӧ����________��
��3��C��D�Ļ�ѧ����ʽ________��
��4������˵����ȷ����________��(����)
A��A��O2�ڴ��������·�ӦҲ��������D B���������ý����Ƽ���C���Ƿ�������ˮ
C��������NaHCO3��Һ����C��D��E��ˮ��Һ D��G������NaOH��Һ��ϡ�����з���ˮ��
���𰸡� �Ȼ� �Ӿ۷�Ӧ 2CH3CH2OH+O2![]() 2CH3CHO+2H2O ABD
2CH3CHO+2H2O ABD
�����������⿼���л�����ƶϣ�AΪʯ���ѽ����Ҫ�������Է�������Ϊ28����AΪCH2=CH2��BΪ���壬���ڱ�״���µ��ܶ�Ϊ1.965g��L��1����B��Ħ������Ϊ1.965��22.4g��mol��1=44g��mol��1����BΪCO2��FΪHOCH2CH2OH����ϩ��ˮ��Ӧ�ӳɷ�Ӧ�������Ҵ�����CΪCH3CH2OH��C��D����������Ӧ����DΪ��ȩ���ṹ��ʽΪCH3CHO��D��E����ȩ����������Ӧ����EΪCH3COOH��D��F��Ϊͬ���칹�壬��FΪ��״�������FΪ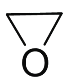 ����1����������������EΪ���ᣬ���еĹ��������Ȼ�����2��B��F�����ķ�Ӧ�ǼӾ۷�Ӧ����3�����������������䷴Ӧ����ʽΪ 2CH3CH2OH+O2
����1����������������EΪ���ᣬ���еĹ��������Ȼ�����2��B��F�����ķ�Ӧ�ǼӾ۷�Ӧ����3�����������������䷴Ӧ����ʽΪ 2CH3CH2OH+O2![]() 2CH3CHO+2H2O ����5��A��CH2=CH2��������һ�������£�����CH3CHO���䷴Ӧ����ʽΪ2CH2=CH2��O2
2CH3CHO+2H2O ����5��A��CH2=CH2��������һ�������£�����CH3CHO���䷴Ӧ����ʽΪ2CH2=CH2��O2![]() 2CH3CHO����A��ȷ��B��CΪ�Ҵ�������������ˮ��Ӧ��Ҳ�����Ҵ���Ӧ������������ˮ��Ӧ�������Ǹ����Ρ��ۡ��죬���������Ҵ���Ӧ�Ƚϻ����������Ƴ����Ҵ��ĵײ�������ͬ�����Լ����Ҵ����Ƿ���ˮ����B��ȷ��C���Ҵ�����ȩ����NaHCO3��Ӧ����������NaHCO3��Ӧ����CO2����˲��ܼ����Ҵ�����ȩ����C����D������G�Ľṹ��ʽ��G�к�������������ڼ�����з���ˮ�⣬��D��ȷ��
2CH3CHO����A��ȷ��B��CΪ�Ҵ�������������ˮ��Ӧ��Ҳ�����Ҵ���Ӧ������������ˮ��Ӧ�������Ǹ����Ρ��ۡ��죬���������Ҵ���Ӧ�Ƚϻ����������Ƴ����Ҵ��ĵײ�������ͬ�����Լ����Ҵ����Ƿ���ˮ����B��ȷ��C���Ҵ�����ȩ����NaHCO3��Ӧ����������NaHCO3��Ӧ����CO2����˲��ܼ����Ҵ�����ȩ����C����D������G�Ľṹ��ʽ��G�к�������������ڼ�����з���ˮ�⣬��D��ȷ��