��Ŀ����
1����Ϣʱ�������Ĵ������������Ի��������˼������в��ij�����Ϊ����ѧ��̽��С�齫һ����������·������õ���70% Cu��25% Al��4% Fe������Au��Pt�Ƚ����Ļ�������Ƴ������Ʊ�����ͭ�������������·�ߣ�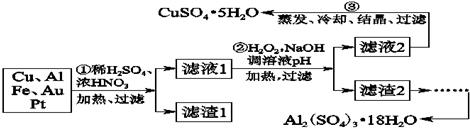
��ش��������⣺
��1���ڢٲ�Cu���ᷴӦ�����ӷ���ʽΪCu+4H++2NO3-$\frac{\underline{\;\;��\;\;}}{\;}$Cu2++2NO2+2H2O��3Cu+8H++2NO3-$\frac{\underline{\;\;��\;\;}}{\;}$3Cu2++2NO��+4H2O���õ�����1����Ҫ�ɷ�ΪAu��Pt��
��2���ڢڲ���H2O2�������ǽ�Fe2+����ΪFe3+������ҺpH��Ŀ����ʹFe3+��Al3+���ɳ�����
��3���õڢ۲�����CuSO4•5H2O�Ʊ���ˮCuSO4�ķ����Ǽ�����ˮ��
��4��������2��ȡAl2��SO4��3•18H2O��̽��С����������ַ�����
�ף�����2$\stackrel{H_{2}SO_{4}}{��}$���Һ����������ȴ���ᾧ������Al2��SO4��3•18H2O
�ң�����2$\stackrel{H_{2}SO_{4}}{��}$���Һ$��_{����}^{����AI��}$��Һ����������ȴ���ᾧ������Al2��SO4��3•18H2O
����$��_{����}^{NaOH��Һ}$��Һ$\stackrel{H_{2}SO_{4}}{��}$��Һ����������ȴ���ᾧ������Al2��SO4��3•18H2O
�������ַ����У����������У�ԭ���Ǽ����ò�Ʒ�к��н϶�Fe2��SO4��3���ʣ�
��ԭ�������ʽǶȿ��ǣ��ҷ�����������
��5��̽��С���õζ����ⶨCuSO4•5H2O��Mr=250��������ȡa g�������100mL��Һ��ÿ��ȡ20.00mL�������������Ӻ���c mol•L-1 EDTA��H2Y2-������Һ�ζ����յ㣬ƽ������EDTA��Һ��b mL���ζ���Ӧ���£�Cu2++H2Y2-�TCuY2-+2H+��д������CuSO4•5H2O���������ı���ʽw=$\frac{cmol•{L}^{-1}��b��1{0}^{-3}L��250g•mo{l}^{-1}��5}{ag}��100%$��
���� ϡ���ᡢŨ�����������ȣ�Cu��Al��Fe������Ӧ����Cu2+��Al3+��Fe2+����Au��Pt����Ӧ����������1 �ijɷ���Pt��Au����Һ1�е�������Cu2+��Al3+��Fe2+��Fe3+���ڢڲ���H2O2�������ǰ�Fe2+����ΪFe3+�������������ŵ��Dz��������ʣ�����Ի���������Ⱦ������ҺpH��Ŀ����ʹFe3+��Al3+�γɳ�����������Һ2�ijɷ���Cu2+���������ᾧ�ɵõ�CuSO4•5H2O���壬����2�ijɷ�Ϊ���������������������������м�NaOH����Al��OH��3��Ӧ����NaAlO2��������Һ�м�H2SO4����Al2��SO4��3����������ȴ���ᾧ�����˿ɵ����������壻
��1��ϡ���ᡢŨ�����������ȣ�Cu��Al��Fe������Ӧ����Cu2+��Al3+��Fe2+����������1 �ijɷ���Pt��Au����Һ1�е�������Cu2+��Al3+��Fe2+��
��2��������������������ұ���ԭΪˮ������������Ⱦ������������������Ϊ���������ڳ�����ȥ��������ҺPHĿ���������Ӻ�������ȫ���������ڢڲ���H2O2�������ǰ�Fe2+����ΪFe3+�������������ŵ��Dz��������ʣ�����Ի�������Ⱦ������ҺPH��Ŀ����ʹFe3+��Al3+�γɳ�����������Һ2�ijɷ���Cu2+������2�ijɷ�Ϊ��������������������
��3���ڢ۲�����ˮ����ͭ�Ʊ�����ͭ�ķ���Ӧ���������м�����ˮ��
��4������ʵ�鷽�����̷����Ʊ��������Ƿ������ʣ�ʹ�õ��Լ����ã�ԭ�ϵ������ʣ�ԭ�����������ط����жϣ�
��5�����ݵζ�ʵ��ͷ�Ӧ���ӷ���ʽ����õ����ζ�ʵ�����������ݱ���Һ���ĵĶ��ٽ��з����жϣ�
��� �⣺��1��ϡ���ᡢŨ�����������ȣ�Cu��Al��Fe������Ӧ����Cu2+��Al3+��Fe2+����������1�ijɷ���Pt��Au����Һ1�е�������Cu2+��Al3+��Fe2+���ڢٲ�Cu���ᷴӦ�����ӷ���ʽΪ��Cu+4H++2NO3-$\frac{\underline{\;\;��\;\;}}{\;}$Cu2++2NO2+2H2O��3Cu+8H++2NO3-$\frac{\underline{\;\;��\;\;}}{\;}$3Cu2++2NO��+4H2O��
�ʴ�Ϊ��Cu+4H++2NO3-$\frac{\underline{\;\;��\;\;}}{\;}$Cu2++2NO2+2H2O��3Cu+8H++2NO3-$\frac{\underline{\;\;��\;\;}}{\;}$3Cu2++2NO��+4H2O��Au��Pt��
��2���ڢڲ���H2O2�������ǽ�Fe2+����ΪFe3+��2Fe2++H2O2+2H+=2Fe3++2H2O��������ҺPH�����Ӻ�������ȫ����������˵õ���������������������������Һ����ͭ���ʴ�Ϊ����Fe2+����ΪFe3+��Fe3+��Al3+��
��3�����������ֽ⣬�����ü�����ˮ�ķ�����ȥ�ᾧˮ���ʴ�Ϊ��������ˮ��
��4���Ʊ�����������ļס��ҡ������ַ����У�������������ֻ�����������������������������ȴ���ᾧ�����˵õ��������������л��д������������ʣ��ҷ������������м�H2SO4������Fe2��SO4��3��Al2��SO4��3���ټ�Al�ۺ�Fe2��SO4��3����Al2��SO4��3����������ȴ���ᾧ�����˿ɵ����������壻���������������м�NaOH��Al��OH��3��Ӧ����NaAlO2��������Һ�м�H2SO4����Al2��SO4��3����������ȴ���ᾧ�����˿ɵ����������壻����ԭ�����ýǶȿ��Ƿ��������������Ϊ���ӵ�NaOH���Ʊ���Al2��SO4��3��ԭ�����û�й�ϵ�����ԭ���˷ѣ������������ַ����У������Ƶõ������������л��д������������ʣ������У���ԭ�������ʺ��Ƿ�������ʿ���֪���ҷ������������ʴ�Ϊ���ף������ò�Ʒ�к��н϶�Fe2��SO4��3���ʣ��ң�
��5��ȡa g�������100mL��Һ��ÿ��ȡ20.00mL�������������Ӻ���c mol•L-1 EDTA��H2Y2-������Һ�ζ����յ㣬ƽ������EDTA��Һ6mL���ζ���Ӧ���£�Cu2++H2Y2-=CuY2-+2H+��ͭ�������ʵ����ͱ�Һ���ʵ�����ͬ=cmol/L��b��10-3L=bc��10-3mol������Ԫ���غ�õ���
��20ml��Һ�к��е�CuSO4•5H2O���ʵ���Ϊbc��10-3mol��100ml��Һ�к�bc��10-3mol��5=5bc��10-3mol��
����CuSO4•5H2O���������ı���ʽ=$\frac{cmol•{L}^{-1}��b��1{0}^{-3}L��250g•mo{l}^{-1}��5}{ag}$��100%���ʴ�Ϊ��$\frac{cmol•{L}^{-1}��b��1{0}^{-3}L��250g•mo{l}^{-1}��5}{ag}$��100%��
���� ���⿼�����ӷ���ķ�����ʵ����ƣ��Լ�ѡ���к͵ζ��ļ�������ķ������������ʵ����������ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�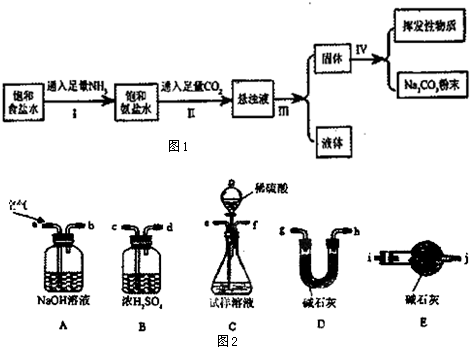
�ڳ����£��й����ʵ��ܽ��Ϊ��
| ���� | NH4Cl | NaHCO3 | Na2CO3 | NaCl |
| �ܽ��/g | 37.2 | 9.6 | 21.5 | 36.0 |
��2������I��II�ܷ�Ӧ�����ӷ���ʽΪNa++NH3+CO2+H2O�TNaHCO3��+NH4+��
��3������I��II���ܵߵ���ԭ������NH3�ڱ���NaCl��Һ���ܽ�����CO2����ͨNH3����Һ�ʼ��ԣ���������CO2������NaHCO3�����ɣ�����Ӧ��ͨ��NH3��
��4���������õ�̼���Ʒ�ĩ�Ƿ���NaHCO3����ʵ�鷽���ǣ�д���������衢�����ۣ���ȡ�����������Թܣ����ȣ�������������ͨ�����ʯ��ˮ����ʹ����ʯ��ˮ����ǣ�֤����NaHCO3��
��5��Ϊ�˲ⶨ����ȡ����Ĵ��ȣ���������ֻ��̼�����ƣ�����С���ʵ�鲽��Ϊ��
i��ʹ������װ����װʵ��װ�ã������������
ii����ȡWg��Ʒ����Cװ�õ���ƿ�У�����������ˮ�ܽ�
iii������Dװ�õ�����ΪW1 g
iv���ӷ�Һ©������ϡ���ᣬֱ�����ٲ�������Ϊֹ
v����a����������һ�����Ŀ������ٴγ���Dװ������ΪW2 g
vi���ظ�����v�IJ�����ֱ��Dװ�õ��������ٸı䣬�Ƶ�Dװ�õ�����ΪW3 g
��������ʵ��ش��������⣺
�ٵ�i����ʹ������װ�����ӵĽӿ�˳��Ϊ����b������e����f������c����d������g����h��[��h����g��]����i����
�ڵڶ���ʢ��ʯ��װ�õ������Ƿ�ֹ��������D�м�ʯ�Ҹ���ʵ�飮
�۲�����̼���ƺ�̼�����Ƶ����ʵ���֮��Ϊ$\frac{{w}_{3}-{w}_{1}}{44}$mol��
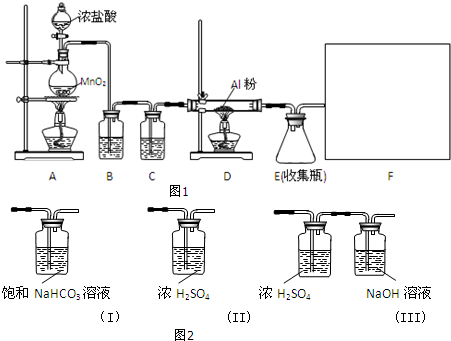
��1��Aװ���з�����Ӧ�Ļ�ѧ����ʽΪ��MnO2+4HCl��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+Cl2��+2H2O��
��2��װ��B�������dz�ȥCl2�л��е�HCl���壮
��3��������ΪF��ʵ�߷���ѡ����ʵ�װ�ã�ͼ2��III��
��4��AlCl3����ʪ��������������������ԭ����AlCl3��ˮˮ�⣬Al3++3H2O?Al��OH��3+3H+���������Ȼ��������ڿ������γ�������������ӷ���ʽ˵������
��5���Ʊ���Ӧ��������Ũ���½���ֹͣ��Ϊ�ⶨ��Ӧ����Һ�������Ũ�ȣ���С��ͬѧ�������к͵ζ����ⶨ�������������£�����ȷ��ȡ������Һϡ��һ���ı�������Ϊ������ƽ������4�εζ���ʵ��������NaOH��ҺŨ��Ϊ0.2000mol•L-1��������ʵ���¼�����ݴ�����
 | 1 | 2 | 3 | 4 |
| V�� ��Ʒ �� | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH������������ | 0.00 | 0.50 | 0.70 | 1.00 |
| V��NaOH�����ն����� | 22.60 | 22.25 | 22.05 | 23.00 |
���ڱ�ʵ��ĵζ������У����в�����ʹʵ����ƫС����df����д��ţ���
a����ʼ�ζ�ʱ�ζ��ܼ��촦�������ݣ��ζ�����������ʧ
b��δ�ñ�NaOH��Һ��ϴ�ζ���
c����ƿ�м������������ټ�����ˮ
d����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��
e���ζ�ǰ��ƿ�ô���������ϴ
f���۲����ʱ���ζ�ǰ���ӣ��ζ�����
��6����ҵ�ϳ������Ʊ����������֣�
a���ɽ�������������ȡ��2Al+3Cl2$\frac{\underline{\;\;��\;\;}}{\;}$2AlCl3
b������ˮ�Ȼ������������ڽ�������ȡ��2Al+6HCl$\frac{\underline{\;\;��\;\;}}{\;}$2AlCl3+3H2��
c����̼�Ȼ�����ȡ��Al2O3+3C+3Cl2 $\frac{\underline{\;\;��\;\;}}{\;}$2AlCl3+3CO
d���������������Ȼ�̼��Ӧ��ȡ��Al2O3+3CCl4�T2AlCl3+3COCl2����������һ����ɫ�綾���壩
���й��������ַ�����������ȷ����CD
A�����ĸ���Ӧ��AlCl3��Ϊ��������
B����Ӧb���Ը�д�����ӷ���ʽ2Al+6H+�T2Al3++3H2��
C����Ӧc��ÿ����2mol AlCl3ʱת�Ƶ�����Ϊ6NA��NA���������ӵ�������ֵ��
D����Ӧc��d������ɴ�����Ⱦ��
 ��Ȼά����P���ṹ��ͼ�����ӽṹ��RΪ���������������ڻ��������У�����һ��Ӫ��������������ά����P��������ȷ���ǣ�������
��Ȼά����P���ṹ��ͼ�����ӽṹ��RΪ���������������ڻ��������У�����һ��Ӫ��������������ά����P��������ȷ���ǣ�������| A�� | ������ˮ��Ӧ����1 mol��������������ˮ��Ӧ����6 mol Br2 | |
| B�� | ����NaOH��Һ��Ӧ��1 mol�����ʿ���5 mol NaOH��Ӧ | |
| C�� | һ��������1 mol�����ʿ���H2�ӳɣ���H2�����Ϊ6 mol | |
| D�� | ά����P�ܷ���ˮ�ⷴӦ |
| A�� | �����£�1.6g�������ͳ�����ɵĻ�����к�����ԭ�ӵ���ĿΪ0.1NA | |
| B�� | ��״���£�22.4 L�����й��ۼ���ĿΪ19NA | |
| C�� | 1mol�ǻ���1mol����������������������Ϊ9 NA | |
| D�� | �ڹ���������ˮ�ķ�Ӧ�У�ÿ����0.1mol������ת�Ƶ��ӵ���ĿΪ0.4NA |
 ��273K����0�棩ʱ������ͼ��ʾ������ܱ�����A�г���0.5g H2ʱ����������ڵ�ѹǿΪ1.01��105Pa���ɴ˿����ж�A���������ԼΪ5.6L������A�г������O2��ѹǿҲ��1.01��105pa��������O2������8g������A�����г������C0���壬ʹ������ѹǿ�ﵽ3.03��105Pa�������C0�����������21g��
��273K����0�棩ʱ������ͼ��ʾ������ܱ�����A�г���0.5g H2ʱ����������ڵ�ѹǿΪ1.01��105Pa���ɴ˿����ж�A���������ԼΪ5.6L������A�г������O2��ѹǿҲ��1.01��105pa��������O2������8g������A�����г������C0���壬ʹ������ѹǿ�ﵽ3.03��105Pa�������C0�����������21g��