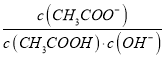��Ŀ����
����Ŀ�����ֶ���������Ԫ��X��Y��Z��W��ԭ���������������������X��Y��Z����Ԫ����ɣ�25��ʱ��0.01mol/L ����Һ�е�c(OH-)/c(H+)=1010��Z��W ͬ���ڣ���W������ϼ�����ͻ��ϼ۵Ĵ�����Ϊ4������˵������ȷ����
A. �����ʵ����Ļ�����Z2Y2 ��Z2W�������Ӹ�����ͬ
B. ԭ�Ӱ뾶X
C. մ��W�ĵ��ʵ��Թܿ��þƾ�ϴ��
D. ���⻯����ȶ���Y
���𰸡�A
�����������ֶ���������Ԫ��X��Y��Z��W��ԭ���������������������X��Y��Z����Ԫ����ɣ�25��ʱ��0.01molL-1����Һ��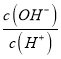 =1010����֪c(OH-)=0.01mol/L�����ΪNaOH�����ԭ��������֪XΪH��YΪO��ZΪNa��Z��Wͬ���ڣ���W���������������۵Ĵ�����Ϊ4�������Ϊ+6�ۣ���֪WΪS��������������֪��XΪH��YΪO��ZΪNa��WΪS��A. �������ƺ������е������ӷֱ�Ϊ���������Ӻ������ӣ������ʵ����Ļ�����������ƺ����Ƶ������Ӹ�����ͬ����A��ȷ��B�����Ӳ�Խ�࣬ԭ�Ӱ뾶Խ��ͬ���ڴӴ�������ԭ�Ӱ뾶��С����ԭ�Ӱ뾶��X��Y��W��Z����B����C��S���ھƾ���������CS2��ӦѡCS2ϴ�ӣ���C����D���ǽ�����Խǿ����Ӧ�⻯��Խ�ȶ������⻯����ȶ��ԣ�Y��W����D��������ѡA��
=1010����֪c(OH-)=0.01mol/L�����ΪNaOH�����ԭ��������֪XΪH��YΪO��ZΪNa��Z��Wͬ���ڣ���W���������������۵Ĵ�����Ϊ4�������Ϊ+6�ۣ���֪WΪS��������������֪��XΪH��YΪO��ZΪNa��WΪS��A. �������ƺ������е������ӷֱ�Ϊ���������Ӻ������ӣ������ʵ����Ļ�����������ƺ����Ƶ������Ӹ�����ͬ����A��ȷ��B�����Ӳ�Խ�࣬ԭ�Ӱ뾶Խ��ͬ���ڴӴ�������ԭ�Ӱ뾶��С����ԭ�Ӱ뾶��X��Y��W��Z����B����C��S���ھƾ���������CS2��ӦѡCS2ϴ�ӣ���C����D���ǽ�����Խǿ����Ӧ�⻯��Խ�ȶ������⻯����ȶ��ԣ�Y��W����D��������ѡA��

 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д� �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д� �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�����Ŀ��2017�꣬�п�Ժ������ѧ�����о���̼��ԴС�������������ô��������о��飨DNL19T3���」��������о�Ա�Ŷ�ͨ�����һ������Na-Fe3O4/HZSM-5��ܸ��ϴ������ɹ�ʵ����CO2ֱ�Ӽ�����ȡ������ֵ���͡����о��ɹ�������Ϊ��CO2��ת�������ͻ���Խ�չ����
(1) ��ҵ����β���е�CO2������֮һ�ǰ�ˮ��Һ���ռ��������������ǽ�������ȴ��15.5��26.5����ð�ˮ���չ�����CO2���÷�Ӧ�Ļ�ѧ����ʽΪ_____________�����ð�ˮ����ǰ����������ȴ��15.5��26.5��Ŀ���ԭ����_________________��
(2) ���о��ɹ���CO2��ȡ������ֵ���͵�·�����Ƚ�CO2����ˮú����Ӧ����CO��Ȼ����CO��H2��Ӧ���������������������ռ�����������İٷֱȽ�80%��
�� ��֪��H2 (g)��![]() O2 (g) === H2O(l) ��H1= ��285.8 kJ��mol-1
O2 (g) === H2O(l) ��H1= ��285.8 kJ��mol-1
C8H18(l)��![]() O2(g) === 8CO2(g)��9H2O(l) ��H3= ��5518 kJ��mol-1
O2(g) === 8CO2(g)��9H2O(l) ��H3= ��5518 kJ��mol-1
��д��25�桢101kPa�����£�CO2��H2��Ӧ��������(��C8H18��ʾ)���Ȼ�ѧ��
��ʽ__________________________��
�� ��һʵ�������г���һ������CO2��H2�����������ǡ����ȫ��Ӧ���Ҳ���ֻ����C5���ϵ��������ʺ�ˮ����CO2��H2�����ʵ���֮�Ȳ�����________��
(3) ������(CH3OCH3)��ʮ����ֵ�ߣ�ȼ��β������Ⱦ���٣��ɴ�����͡�CO��CO2��ϼ���ķ�������һ����Ӧ���н��ϳ���ֱ��ת��Ϊ�����ѣ���������4����Ӧ��
CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g) ��
CH3OH(g)��H2O(g) ��
CO2(g)��H2(g)![]() CO(g)��H2O(g) �� ��
CO(g)��H2O(g) �� ��
CO(g)��2H2(g)![]() CH3OH(g) ��
CH3OH(g) ��
2CH3OH(g)![]() CH3OCH3(g)��H2O(g) ��
CH3OCH3(g)��H2O(g) ��
�� ��֪��Ӧ����ij�¶��µ�ƽ�ⳣ��ΪK=400�����¶��£���һ�����ܱ������м���CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
���� | CH3OH | CH3OCH3 | H2O |
Ũ��/��mol��L��1�� | 0.44 | 0.6 | 0.6 |
��ʱ�����淴Ӧ���ʵĴ�С��v(��) ______ v(��) ���>������<������)��
������CH3OH��10 min��Ӧ�ﵽƽ�⣬��ʱ���ڷ�Ӧ����v(CH3OH)��_____��
�� ij����С���ڷ�Ӧ�¶�503K��543K����Ӧѹǿ�ֱ���3��4��5��6��7MPa��n(CO)/n(CO+CO2)=0.5������¼����˶����ѵ�ƽ������(������ʵ�ʲ����뷴Ӧ������֮��)�������ͼ1��ʾ��

a~e���߷ֱ��Ӧ��ͬ��ѹǿ��������e��Ӧ��ѹǿ��______��������___________��
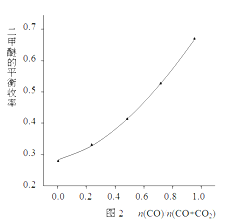
�ÿ���С���ڷ�Ӧ�¶�523K����Ӧѹǿ5MPa�������£����պϳɷ�Ӧ�Ļ�ѧ�����Ƚ��ϣ��ı�n(CO)/n(CO+CO2)�ı����������ѵ�ƽ�����ʵı仯������ͼ2��ʾ��
��ͼ2��֪�������ѵ�ƽ����������n(CO)/n(O+CO2)��ֵ�������������������ԭ����________________________________________________��