��Ŀ����
����Ŀ��ijͬѧ��Ҫ����450 mL 0.5 mol��L��1��NaOH��Һ����ش��������⣺
��1����ʵ��������õ��IJ��������У�����������Ͳ���ձ���______________����ͷ�ιܡ��Լ�ƿ��
��2����������ƽ����ʱ��Ӧ��NaOH����________��������ȡ�Ĺ�������Ϊ_______��
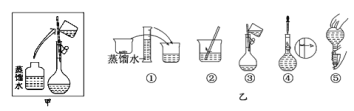
��3������ʱ������������ͼ��ʾ�����ͼ����Ӧ����ͼ�е�___(��ѡ����ĸ)֮�䡣
A������ڡ� B������ۡ� C������ܡ� D�������
��4�����ƹ�����ϴ���ձ���������2��3�ε�Ŀ����______________________��
��5�����ݵμ�����ˮʱ�������������˿̶��ߣ������ķ�����______________��
��6����ͬѧʵ������NaOH��Һ��Ũ��Ϊ0.6 mol��L��1��ԭ�������____(�����)��
a��������������
b��ϴ��������ƿ�в���������ˮ
c������NaOH����ʱ�������ˡ��������
d������ʱ���ӿ̶���
e������ҡ�Ⱥ���Һ����ڿ̶����ּ�������ˮ�����̶���
f���ܽ������ձ���Һ��δϴ��
g������ǰ��Һδ������ȴ
���𰸡�500 mL����ƿ С�ձ� 10.0g C ��֤����ȫ��ת��������ƿ�� �������� a d g
��������
(1)��������һ�����ʵ���Ũ����Һ��һ�㲽�裬ѡ����Ҫ��������
(2)����450mL��Һ����Ҫѡ��500mL����ƿ������n=cV��m=nM�������Ҫ�������������ƹ����������
(3)��ͼ��ʾ�IJ���Ϊ��Һϴ�Ӻ�������ƿ�ڼ�ˮ��������Ʋ����ж���
(4)ת����Һʱ��Ҫ�Ѳ������ձ��е�����ȫ��ת������ƿ��
(5)������Һʱ���ִ��������Ҫ�������ƣ�
(6)�����������������ʵ����ʵ�������Һ�����Ӱ�죬����c=![]() ������������
������������
(1)����һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢����(��ȡ)���ܽ�(ϡ��)����ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ��õ���������������ƽ��ҩ�ס���Ͳ���ձ�����������500mL����ƿ����ͷ�ιܣ����Ի�ȱ�ٵ�������500mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��500mL����ƿ��
(2)ʵ����û��450mL������ƿ������ʱ��Ҫѡ��500mL����ƿ��ʵ�������Ƶ���450mL0.5 mol/LNaOH��Һ����Ҫ�������ƹ���õ�����Ϊ��m=40g/mol��0.5 mol/L��0.5L=10.0g���������ƾ���ǿ��ʴ�ԣ������׳��⣬����ʱӦ�÷����ձ��п��ٳ������ʴ�Ϊ���ձ���10.0g��
(3)��ͼ��ʾ�IJ���Ϊ��Һϴ�Ӻ�������ƿ�ڼ�ˮ��Ӧ��ת���붨��֮�䣬��Ӧ�ڢۺ͢�֮�䣬��ѡ��C��
(4)���ձ��е���Һת�Ƶ�����ƿʱ���ձ��л��в������������ƣ�Ϊ�˱�֤����ȫ��ת��������ƿ�У�Ҫϴ���ձ�2��3�Σ�����ϴҺת������ƿ���ʴ�Ϊ����֤����ȫ��ת��������ƿ�У�
(5)�ڶ��ݵμ�����ˮʱ�������������˿̶��ߣ���Һ�����ƫ��Ũ��ƫС��Ҫ�������ƣ��ʴ�Ϊ���������ƣ�
(6)��ͬѧʵ������NaOH��Һ��Ũ��Ϊ0.6molL-1��Ũ��ƫ�ߡ�a��������մ�����ʣ���ȡ���ʵ�����ƫ�����ʵ����ʵ���ƫ����Һ��Ũ��ƫ��aѡ��b������ƿ��ԭ����������ˮ�������ʵ����ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣬��b��ѡ��c������NaOH����ʱ������������������������Ϊʵ�������û���õ����룬���Զ����ʵ����ʵ���������Ӱ�죬��ҺŨ�Ȳ��䣬��c��ѡ�� d������ʱ�����ӿ̶��ߣ�������Һ�����ƫС����Һ��Ũ��ƫ��dѡ��e������ҡ�Ⱥ���Һ����ڿ̶����ּ�������ˮ�����̶��ߣ�������Һ���ƫ��Ũ��ƫС����e��ѡ��f���ܽ������ձ���Һ��δϴ�ӣ����²������������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���f��ѡ��g��ת����Һʱδ����ȴ����ȴ��Һ���½�����Һ���ƫС����ҺŨ��ƫ�ߣ���gѡ���ʴ�Ϊ��adg��

 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�