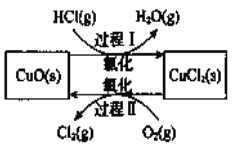
��Ŀ����
����Ŀ�����ⶨ�������Ȼ��Ƶ�С�մ��̬��Ʒ��NaHCO3���������������������ַ�����
����һ��
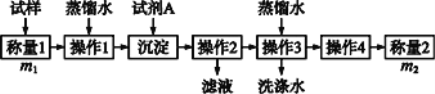
��������
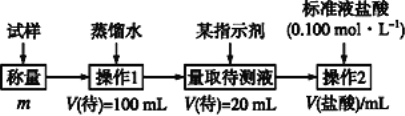
��������
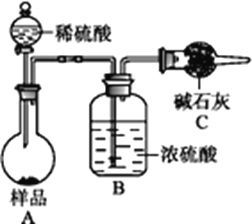
�����ģ���ʹ�û�ѧ�Լ�,ʹ��ʵ���ҳ���������
��Ҫ��ش���������:
��1������һ:�����������Լ�A��___________(��дA�Ļ�ѧʽ),�ɽ�HCO3-ת��Ϊ���������ء�����1��2��3��4�������������ܽ⡢____��ϴ�Ӻ���(���);
��2��������:�ڲ���1�����õ��IJ���������,�����ձ�������������ͷ�ι���,����Ҫ�õ�����__________,Ӧѡ�������ָʾ��;
��3���ڷ������м������Ʒ��NaHCO3����������Ϊ_____________;
��4���ڷ�������,�������õ�ʵ��װ��,���˳�����Ʒ������,����ⶨ��ʵ��������_____________________;
��5����ϸ�����������е�ʵ��װ��,���ɴ˲�õ�����������ʵ����,���п���ƫ��Ҳ�п���ƫ��,ƫ�ߵ�ԭ�������_________,ƫ�͵�ԭ�������__________(�����ּ���);
��6�������ĵ�ʵ��ԭ����________________(�û�ѧ����ʽ��ʾ)��
���𰸡�Ca(OH)2��Ba(OH)2 ���� 100 mL����ƿ 0.042V/m��100% װ�м�ʯ�ҵĸ������ʵ��ǰ������� ��ʯ�ҿ��ܻ������տ����е�ˮ������CO2���� װ���ڻ����沿��CO2���� 2NaHCO3![]() Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2��
��������
����һ��(ͼ��)��̬��Ʒ���Լ�Aʹ̼��������ɳ������پ������ˡ�ϴ�ӡ���������������������Ӷ�����̼�غ����̼�����Ƶ�������������������������
��������(ͼ��)��̬��Ʒ��ˮ�ܽ��100ml��Һ��ȡ20ml��ָʾ�����ñ�������еζ����Ӷ������̼�����Ƶ�����������
��������(ͼ��)��̬��Ʒ��ϡ�������ܽ⣬�پ���Ũ�������ü�ʯ���������ɵĶ�����̼���壬���ݶ�����̼����������̼�����Ƶ�������������������������
�����ģ�����������ѧ�Լ�����ֻ����̼�����Ƶ����ȷֽ��ˣ����ù��巴Ӧǰ������������̼�����Ƶ�����������
(1)��HCO3-��Ӧ�Ʋ����������Լ���Ca(OH)2�� Ba(OH)2������1��2��3��4�������������ܽ⡢���ˡ�ϴ�ӡ����
(2)����������1�IJ������ܽ⡢ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ��õ��������в��������ձ�����Ͳ����ͷ�ιܡ�100 mL����ƿ������100mL������ƿ��
(3)���������������ɼ����20mL�Ĵ���Һ��̼�����Ƶ����ʵ�����ԭҺ�Ǵ���Һ��5�������ԣ���Ʒ��NaHCO3����������ΪV(HCl)��10-3��0��100��5��84/m��100%=![]() ��
��
(4)���������ò���������̼������������̼�����Ƶ�����������������Ҫ����װ�м�ʯ�ҵĸ������ʵ��ǰ���������
(5)ƫ�ߵ�ԭ���Ǽ�ʯ�һ������տ����еĶ�����̼��ˮʹ��������ƫ�͵�ԭ����װ���л��в����Ķ�����̼δ�����գ�
(6)����������ѧ�Լ�����ֻ����̼�����Ƶ����ȷֽ��ˣ����ù��巴Ӧǰ������������̼�����Ƶ�������������ѧ����ʽ��2 NaHCO3![]() Na2CO3+H2O +CO2����
Na2CO3+H2O +CO2����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�