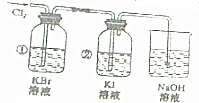
��Ŀ����
13����֪1molij�л��ﺬ̼ԭ��nmol����ǡ����ȫȼ��ʱ����1.5n mol��������ش��й����⣺��1�����л���Ļ�ѧʽ����Ϊ����CnH2n����CnH2n+2O����-����-������n��ʾ�����ڵ�̼ԭ����������Ϊ�л��ֻ�ѧʽ����֣����������Ҫ�۷֣���
��2����4.4g���л��R�����������Ʒ�Ӧ���ռ���H2560ml������£����ɸ��л�������������м��֣�8
��3����д���Ը��л��R����ѧʽ���ƶϹ����л���R����ɷ���CnH2n+2O����֪4.4gR���������Ʒ�Ӧ����������Ϊ$\frac{0.56}{22.4}$=0.025mol
��R�����к��ǻ�0.025��2=0.05mol
���й�ϵʽ��4.4/0.05=14n+2+16n=5
��R�Ļ�ѧʽ��C5H12O��
���� ��1����֪1molij�л��ﺬ̼ԭ��n mol����ȫȼ��ʱֻ����CO2��H2O��˵������C��HԪ�أ����ܺ���OԪ�أ�������1.5n mol���ɷ�Ӧ��ϵC��O2��CO2��4H��O2��2H2O��֪���л�����H����0.5n��������Ϊ��������������C��Hԭ����Ŀ֮��=n��0.5n��4=n��2n������ͨʽ����ΪCnH2n����������Ԫ�أ����Hԭ�ӱ���C���ļ۽ṹ����֪ͨʽΪCnH2n+2O��
��2����4.4g���л��R�����������Ʒ�Ӧ���ռ���H2 560mL����״������˵��ͨʽΪCnH2n+2O������11���ǻ������ݷ�Ӧ��ϵʽ2CnH2n+2O��H2����ȷ�������ʽ����������ͬ���칹�壬���õ�Ч���ж����ڴ���ͬ���칹����Ŀ��
��� �⣺��1����֪1molij�л��ﺬ̼ԭ��n mol����ȫȼ��ʱֻ����CO2��H2O��˵������C��HԪ�أ����ܺ���OԪ�أ�������1.5n mol���ɷ�Ӧ��ϵC��O2��CO2��4H��O2��2H2O��֪���л�����H����0.5n������
��Ϊ��������������C��Hԭ����Ŀ֮��=n��0.5n��4=n��2n������ͨʽ����ΪCnH2n��
��������Ԫ�أ����Hԭ�ӱ���C���ļ۽ṹ��n��Cԭ���������2n+2��Hԭ�ӣ���֪ͨʽΪCnH2n+2O��
�ʴ�Ϊ��CnH2n��CnH2n+2O��
��2����4.4g���л��R�����������Ʒ�Ӧ���ռ���H21.12L����״������˵��ͨʽΪCnH2n+2O������1���ǻ���n��H2��=$\frac{0.56L}{22.4L/mol}$=0.025mol���ɹ�ϵʽ2CnH2n+2O��H2��֪n��CnH2n+2O��=0.025mol��2=0.05mol����M��CnH2n+2O��=$\frac{4.4g}{0.05mol}$=88g/mol����14n+2+16=88����ã�n=5��qi ����ʽΪC5H12O��
��Ϊ-OHȡ��CH3CH2CH2CH2CH3��Hԭ���γɵĴ�����3�ֽṹ��
��Ϊ-OHȡ��CH3CH2CH��CH3��2��Hԭ���γɵĴ�����4�ֽṹ��
��Ϊ-OHȡ��C��CH3��4��Hԭ���γɵĴ�����1�ֽṹ���ô�����3+4+1=8�֣�
�ʴ�Ϊ��8��
��3�����ݣ�2���ķ�����֪�����л���R����ɷ���CnH2n+2O����֪4.4g R���������Ʒ�Ӧ����������Ϊ$\frac{0.56}{22.4}$=0.025mol
��R�����к��ǻ�0.025��2=0.05mol
���й�ϵʽ��4.4/0.05=14n+2+16n=5
��R�Ļ�ѧʽ��C5H12O��
�ʴ�Ϊ���л���R����ɷ���CnH2n+2O����֪4.4g R���������Ʒ�Ӧ����������Ϊ$\frac{0.56}{22.4}$=0.025mol
��R�����к��ǻ�0.025��2=0.05mol
���й�ϵʽ��4.4/0.05=14n+2+16n=5
��R�Ļ�ѧʽ��C5H12O��
���� ���⿼�����л������ʽ���ṹ��ʽ��ȷ������Ŀ�Ѷ��еȣ���ȷ�����л���ṹ������Ϊ���ؼ���ע������ͬ���칹�����дԭ������������ѧ���ķ�����������ѧ����������

| A�� | ��״���£�22.4 L�Ҵ������ķ������ض�ΪNA | |
| B�� | 1mol NO2��������ˮ��Ӧ��ת�Ƶĵ�����Ϊ2NA | |
| C�� | 0.1mol Fe��������ˮ������Ӧ���ɵ�H2������Ϊ0.15NA | |
| D�� | �ڳ��³�ѹ��32g����������ԭ����ĿΪ2NA |
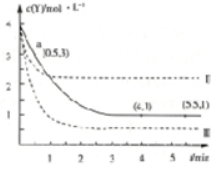
| A�� | ��Ӧ��ʼ��a��ʱ��v��X��=1mol•L-1•min-1 | |
| B�� | 4minʱ������Ӧ���ʣ��� | |
| C�� | ���ߢ��Ӧ��������ı��Ǽ�Сѹǿ | |
| D�� | T��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ1 |
| A�� | 1molNa2O��Na2O2�����������������������3NA | |
| B�� | 1molNa2O2��CO2��H2O�Ļ���ﷴӦ������ת��2NA | |
| C�� | 1molCl2������Fe��Ӧת�Ƶ�����һ��Ϊ3NA | |
| D�� | 1mol•L-1��AlCl3��Һ�У�Cl-����ĿΪ3NA |
| A�� | 1.0L1.0mol•L-1��NaAlO2ˮ��Һ�к��е���ԭ������Ϊ2NA | |
| B�� | ���³�ѹ�£�3.4gNH3�к�NһH����ĿΪ0.6NA | |
| C�� | ����������ˮ��Ӧʱ������0.1mol����ת�Ƶĵ�����Ϊ0.4NA | |
| D�� | ��״���£�33.6LCCl4�к�����ԭ�ӵ���ĿΪ6NA�� |
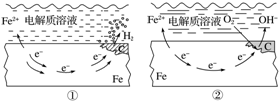
| A�� | ̼���淢��������Ӧ | |
| B�� | ��������ʴ�����ղ���ΪFeO | |
| C�� | �����и�����Ʒ�ĸ�ʴ��ͼ����ʾΪ�� | |
| D�� | ͼ���У�������ӦʽΪO2+4e-+2H2O�T4OH- |