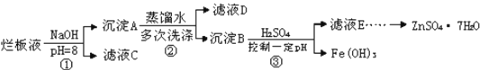
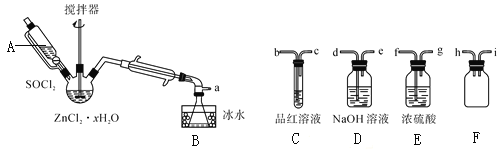
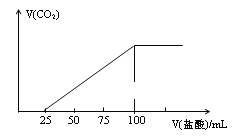
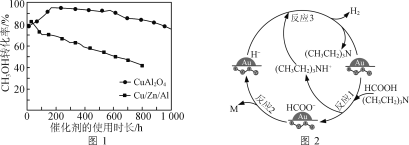
题目内容
【题目】钛(Ti)被称为“未来金属”,广泛应用于国防、航空航天、生物材料等领域。钛的氯化物有如下转变关系:2TiCl3![]() TiCl4↑+TiCl2回答下列问题。
TiCl4↑+TiCl2回答下列问题。
(1)某同学所画基态 Cl-的外围电子排布图为![]() ,这违反了____________
,这违反了____________
(2)从结构角度解释 TiCl3中Ti(III)还原性较强的原因____________。
(3)钛的氯化物的部分物理性质如下表:
氯化物 | 熔点/℃ | 沸点/℃ | 溶解性 |
TiCl4 | -24 | 136 | 可溶于非极性的甲苯和氯代烃 |
TiCl2 | 1035 | 1500 | 不溶于氯仿、乙醚 |
①TiCl4与TiCl2的晶体类型分别是____________。
②TiCl4与SO42-互为等电子体,因为它们____________相同;SO42-中心原子以3s轨道和3p轨道杂化。
(4)Ti的配合物有多种。Ti(CO)6、Ti(H2O)63+、TiF62-的配体所含原子中电负性最小的是__________;Ti(NO3)4的球棍结构如图,Ti的配位数是_____________
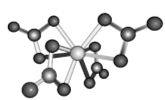
(5)钙钛矿(CaTiO3)是自然界中的一种常见矿物,其晶胞结构如图:
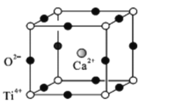
①设N为阿伏加德罗常数的值,计算一个晶胞的质量为______________g.
②假设O2-采用面心立方最密堆积,Ti4+与O2-相切,则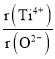 =_________。
=_________。
【答案】泡利原理 +3价Ti外围电子为3d1,失去一个电子后,3d能级处于全空稳定状态 分子晶体;离子晶体 原子总数、价电子总数 H 8 ![]()
![]() -1或0.414
-1或0.414
【解析】
(1)根据原子核外电子排布规律分析;
(2)根据原子核外各个电子层排布处于全满、半满或全空时稳定分析;
(3)①根据分子晶体熔沸点低,易溶于某些溶剂中,而离子晶体熔沸点较高,在有机溶剂中不能溶解分析判断;
②根据等电子体的概念及性质分析判断;
(4)元素的非金属性越弱,元素的电负性就越小;结合图示中与Ti形成共价键的原子数目确定其配位体数目;
(5)①用均摊方法计算一个晶胞中含有的各种元素的原子个数,然后结合摩尔质量与相对原子质量关系计算晶胞质量;
②O2-采用面心立方堆积,则各种离子相对位置可表示为: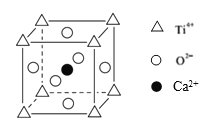 ,面对角线为2个O2-半径与2个Ti4+的半径,晶胞边长为2个O2-半径,据此分析解答。
,面对角线为2个O2-半径与2个Ti4+的半径,晶胞边长为2个O2-半径,据此分析解答。
(1)Cl是17号元素,核外电子排布为2、8、7,Cl原子获得1个电子变为Cl-,根据构造原理,基态Cl-核外电子排布式是1s22s22p63s23p6,3p亚层有3个能量相同的轨道,每个轨道最多容纳2个自旋方向相反的电子,这样排布使离子能量最低,稳定,故基态 Cl-的外围电子排布图为![]() ,而
,而![]() 则违背了泡利不相容原理;
则违背了泡利不相容原理;
(2)Ti是22号元素,外围电子排布是3d24s2,TiCl3中Ti的化合价为+3价,+3价Ti外围电子为3d1,当其再失去一个电子后,3d能级处于全空的稳定状态,因此TiCl3中Ti(III)还原性较强;
(3)根据表格性质可知TiCl4熔沸点低,易溶于有机溶剂,则TiCl4是由分子构成的分子晶体;而TiCl2熔沸点较高,在乙醇、乙醚中不能溶解,说明TiCl2的晶体类型属于离子晶体;
②TiCl4与SO42-的原子总数、价电子总数相等,因此二者互为等电子体;
(4)在Ti的多种配合物Ti(CO)6、Ti(H2O)63+、TiF62-中,配体分别是CO、H2O、F-,其中含有的非金属性元素有C、O、H、F,元素的非金属性:F>O>C>H,元素的非金属性越弱,其电负性就越小,故上述所含非金属元素原子中电负性最小的是H;根据Ti(NO3)4的球棍结构图示可知Ti的配位数是8;
(5)①在一个晶胞中含有Ca2+离子数目是1;含有Ti4+离子数目为8![]() =1,含有的O2-数目为12
=1,含有的O2-数目为12![]() =3,则一个晶胞中含有1个CaTiO3,设NA为阿伏加德罗常数的值,则一个晶胞的质量为
=3,则一个晶胞中含有1个CaTiO3,设NA为阿伏加德罗常数的值,则一个晶胞的质量为![]() =
=![]() g;
g;
②假设O2-采用面心立方最密堆积, Ti4+与O2-相切,则晶胞边长为L=2r(O2-),晶胞的面对角线
Ti4+与O2-相切,则晶胞边长为L=2r(O2-),晶胞的面对角线![]() L=2r(O2-)+2r(Ti4+),故r(O2-)=
L=2r(O2-)+2r(Ti4+),故r(O2-)=![]() L,r(Ti4+)=
L,r(Ti4+)=![]() L-
L-![]() L ,所以
L ,所以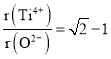 =0.404。
=0.404。

 阳光课堂课时作业系列答案
阳光课堂课时作业系列答案