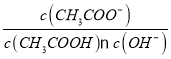��Ŀ����
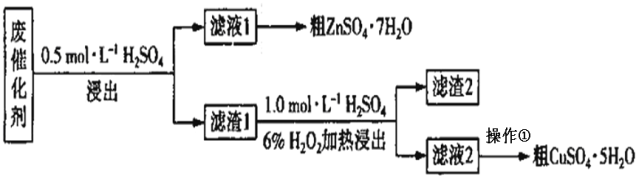
����Ŀ��ij�ϴ�����SiO2��ZnO��ZnS��CuS��ʵ���ҴӸ÷ϴ����л���п��ͭ��һ����������ͼ��ʾ��
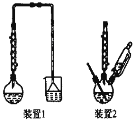
����ʹ�õ�װ����ͼ��ʾ���ش��������⣺
 ����Ũ������ȴ�ᾧ����Ũ������ȴ�ᾧ
����Ũ������ȴ�ᾧ����Ũ������ȴ�ᾧ
(1)��һ�ν�������ѡ��װ�� _______(����1������2��)��ԭ���� ________������1����Ҫ�ɷ���_______��
(2)�ڶ��ν���ʱ���ɵ�����д����Ӧ�����ӷ���ʽ��_______________���ò��輴ʹ�����¶Ȳ���Ҳ���ܻ��и���Ӧ����������֮һ�ǿ�������Ҫ�ɷ֣���������ԭ����_____________________ ��
(3)����Һ2�еõ�CuSO4��5H2O�IJ����� __________ �����ˡ�ϴ�ӡ�
(4)�����������漰����������е��������١���ͬ��������_____________ ��
A.�������������ű����������һ�գ���ˮ���գ���ȡ֭������֮��
B.���������塷�о�����˪�������������û��ϣ�������֮���������Ϸ��Ÿ�����
C.���������ݡ��л�ȡ����أ������µ�����˪��ɨȡ��ˮ��֭���˼���������
D. �����ݸ�Ŀ�����վƵ����칤�գ������ỵ֮�ƣ��Կ�������
(5)���÷ϴ����к�12.8%��CuS��ʵ���г�ȡ15.0g�ϴ������յõ�3.0gCuSO4��5H2O����ͭ�Ļ�����Ϊ __________________ ��
���𰸡�1 ���ж�����H2S���ɣ�װ��2û��β������װ�� SiO2��CuS CuS+H2O2+2H+=Cu2++2H2O+S Cu2+��H2O2�ֽ����O2 ����Ũ������ȴ�ᾧ C 60.0%
��������
�ϴ�������ϡ���ᣬ��һ�ν�����Ҫ������Ӧ��ZnO��H2SO4��ZnSO4��H2O��ZnS��H2SO4��ZnSO4��H2S����Fe3O4��4H2SO4��FeSO4��Fe2(SO4)3��4H2O�����˺���Һ��ZnSO4��FeSO4��Fe2(SO4)3��Ũ���ᾧ�õ���ZnSO47H2O������1����SiO2��CuS����ʢ������1�ķ�Ӧ���м�H2SO4��H2O2��Һ������������ԭ��Ӧ����������ͭ��������2������Ͷ������裬��Һ��������ͭ����Ũ����ᾧ�ɵõ�����ͭ���塣
(1)������Ŀ��ѧ��������֪����һ�ν���������Ӧ��ZnO��H2SO4��ZnSO4��H2O��ZnS��H2SO4��ZnSO4��H2S�������ж�������H2S���ɣ�����������������Һ����β����������װ��2û��β������װ�ã����ѡ1װ�ã� �����е�SiO2��CuS���������ᣬ���������Ӧ���ս�������1��
�ʴ�Ϊ��1�����ж�����H2S���ɣ�װ��2û��β������װ�ã�SiO2��CuS��
(2) �ڶ��ν���ʱ������Ӧ�Ļ�ѧ����ʽ��CuS��H2O2��H2SO4�TCuSO4��S��2H2O����Ӧ�����ӷ���ʽΪ��CuS+H2O2+2H+=Cu2++2H2O+S����Ϊ�������ⲻ�ȶ�����ͭ���Ӵ������»�ֽ������������˼�ʹ�����¶Ȳ���Ҳ���ܻ��и���Ӧ������
�ʴ�Ϊ��CuS+H2O2+2H+=Cu2++2H2O+S��Cu2+��H2O2�ֽ����O2��
(3)��Һ2��Ҫ����������ͭ�����еõ�����ͭ���壬Ӧ���ý��½ᾧ�����������������Ũ������ȴ�ᾧ����ˡ�ϴ�ӵõ�����ͭ���壬
�ʴ�Ϊ������Ũ������ȴ�ᾧ��
(4)A.��ȡ�����صĹ�������������ȡ���ʲ�ѡ��
B.��˪�����Ժ��������Ľ��,���ַ��뷽�����������ʲ�ѡ��
C.����ص��ܽ�����¶ȱ仯���ᴿ�ķ������ȼ�ˮ�ܽ⣬Ȼ�������ᾧ�õ�����ؾ��壬�����1��ͬ����ѡ��
D.�ù����漰����������������ʲ�ѡ��
�ʴ�Ϊ��C��
(5) �ϴ�����Cu�����ʵ���Ϊ15.0g��12.8%��96g/mol��0.02mol��3.0g CuSO4�q5H2O��Cu�����ʺ�����Ϊ3.0g��250g/mol��0.012mol����ͭ�Ļ�����Ϊ![]() ��100%��60%��
��100%��60%��
�ʴ�Ϊ��60%��

����Ŀ�������к͵ζ�
��1������ʽ�ζ�����ȡ20.00 mL����ϡ������Һ������ƿ�У����μ�1��2�η�̪��ָʾ������0.20 mol��L-1NaOH����Һ���еζ���Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡϡ���������Ϊ20��00 mL������ʵ������¼���£�
ʵ����� | ��һ�� | �ڶ��� | ������ |
����NaOH��Һ���/mL | 19.00 | 23.00 | 23.02 |
�������Ũ��ԼΪ_____________(������λ��Ч����)���ζ��ﵽ�յ�ı�־��_____________��
��2��������ʵ���У����в���������������ȷ������ɲⶨ���ƫ�ߵ���_______��
A. �ζ��յ����ʱ���Ӷ���
B. ��ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������ϴ
C. ��ƿˮϴ��δ����
D. ����NaOH����Һʱ��û�е��ܽ�Һ�������¾�ת��������ƿ��
E. ����NaOH����Һʱ������ʱ��������ƿ�Ŀ̶���
F. ��ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ
������1���ڴ���CuSO4��5H2O�����г���������Fe2+�����ᴿʱΪ�˳�ȥFe2+�������������������ʹFe2+����ΪFe3+���������ʿɲ��õ���________
A. KMnO4 �� B. H2O2 ���� C. ��ˮ ���� D. HNO3
��2��Ȼ���ټ����ʵ����ʵ�������ҺpH=4��ʹFe3+ת��ΪFe(OH)3�����Դﵽ��ȥFe3+������ʧCuSO4��Ŀ�ģ�������ҺpH��ѡ�������е�_______��
A. NaOH B. NH3��H2O C. CuO D. Cu(OH)2
��3��������Fe(OH)3���ܶȻ�Ksp=8.0��10-38,Cu(OH)2���ܶȻ�Ksp=3.0��10-20��ͨ����Ϊ��������Һ�е�����Ũ��С��1��10-5 mol��L-1ʱ����Ϊ������ȫ������Һ��CuSO4��Ũ��Ϊ3.0 mol��L-1����Cu(OH)2��ʼ����ʱ��Һ��pHΪ_______��Fe3+��ȫ����ʱ��Һ��pHΪ________����֪ lg5 = 0.7 )