��Ŀ����
19���ظ���أ�K2Cr2O7���׳ƺ췯�أ��ǹ�ҵ������ʵ���ҵ���Ҫ����������֪K2Cr2O7��Һ�д���ƽ��Cr2O72-+H2O?2CrO42-+2H+��
�ش��������⣨��������Һ�е�����Ũ��С��1��10-5 mol•L-1ʱ����������Ϊ������ȫ����
��1���ظ�����Լ���ǩ��Ӧ��ע��ͼ1��A��������ĸ��ţ�
��2������ʱ���ظ������Ũ���ᷴӦ��ʹ�����������ݳ�����д���÷�Ӧ�����ӷ���ʽ��14H++Cr2O72-+6Cl-$\frac{\underline{\;\;��\;\;}}{\;}$2Cr3++3Cl2��+7H2O��
��3����֪AgCl��Ag2CrO4��ש��ɫ����Ksp�ֱ�Ϊ2��10-10��1.12��10-12��������ѧ�У��ⶨ���ȵ�������Һ��Cl-�ĺ�������K2CrO4��ָʾ������AgNO3��Һ�ζ����ζ�������������������AgCl���ﵽ�ζ��յ��ʵ������Ϊ�������һ��AgNO3��Һʱ������ש��ɫ�������ó����ζ���Ҫע���������⣺
��ָʾ�����������ζ��յ�ʱ����Һ�е�CrO42-Ũ��Ϊ2.8��10-3 mol•L-1ʱ���ʣ�
�ڿ�����Һ����ȣ�pH��6.5-10.5֮�䣬���Ϸ�Ӧ����ʽ������ΪʲôpH��6.5ʵ��������ȷ��2CrO42-+2H+
 Cr2O72-+H2O��pHС��c��H+��Ũ�ȴ�ƽ�����ƣ�ʹ�ⶨ�����ȷ��
Cr2O72-+H2O��pHС��c��H+��Ũ�ȴ�ƽ�����ƣ�ʹ�ⶨ�����ȷ����4�������ڸ�Ԫ�صĺ������ߣ����������ж��������°�����ҵ�Ͽɲ�������������⺬Cr2O72-�����Է�ˮ�����ŵ��Ľ��У�������������ҺpH���ߣ�����Fe��OH��3��Cr��OH��3�������Ӷ�ʹ��ˮ�и����������ŷű����±��dz����½������������Ksp�ͽ���������ijŨ���¿�ʼ���������pH������Ũ��Ϊ��ӦpHʱ��Һ���йؽ������Ӳ�����������СŨ�ȣ�[Cr��OH��3��һ��������������]��
| �������� | Ksp | pH��10-1 mol•L-1�� | pH��10-5 mol•L-1�� |
| Fe3+ | 4.0��10-38 | 2.7 | 3.7 |
| Cr3+ | 6.0��10-31 | 4.3 | 5.6 |
��pH�Է�ˮ��Cr2O72-ȥ��Ч�ʵ�Ӱ�������ͼ2��ʾ���������жԽ��ͷ�ˮ�еĸ�������������pH��ΧΪ4.3��5.6��
���� ��1���ظ���ؾ���ǿ�����ԣ�
��2���ظ������Ũ���ᷴӦ�������������ӣ�
��3������Ksp���㱥����Һ�������ӵ�Ũ�ȣ�Ũ��ԽС��Խ�������������ﵽ�ζ��յ�ʱ������Ag2CrO4��ש��ɫ��������
�ٸ���AgCl��KspΪ2��10-10����������ӵ�Ũ�ȣ��ٸ���Ag2CrO4��ש��ɫ����Ksp�����Һ�е�CrO42-Ũ�ȣ�
�ڸ�����Һ�д���2CrO42-+2H+ Cr2O72-+H2O������
Cr2O72-+H2O������
��4���������������ӵõ�������������Cr2O72-���������ӷ���������ԭ��Ӧ����Cr3+�����������ӣ����ݵ����غ���㣻
����ͼ��֪��pHԽС��Cr2O72-��ȥ����Խ�����γ�Cr��OH��3�����ӷ�ˮ�з��룻pHԽ��Cr2O72-��ȥ����ԽС��
��� �⣺��1���ظ���ؾ���ǿ�����ԣ����ظ�����Լ���ǩ��Ӧ��עA���ʴ�Ϊ��A��
��2�����������£��ظ������Ũ���ᷴӦ�������������ӣ��䷴Ӧ�����ӷ���ʽΪ14H++Cr2O72-+6Cl-$\frac{\underline{\;\;��\;\;}}{\;}$2Cr3++3Cl2��+7H2O��
�ʴ�Ϊ��14H++Cr2O72-+6Cl-$\frac{\underline{\;\;��\;\;}}{\;}$2Cr3++3Cl2��+7H2O��
��3������AgCl��Һ��c��Ag+��=$\sqrt{2��1{0}^{-10}}$mol/L������Ag2CrO4��Һ��c��Ag+��=$\root{3}{2��1.12��1{0}^{-12}}$mol/L��c��Ag+��ԽС����Խ�����ɳ��������������ɵij���ΪAgCl��
�ⶨ���ȵ�������Һ��Cl-�ĺ�������K2CrO4��ָʾ������AgNO3��Һ�ζ����ﵽ�ζ��յ�ʱ������Ag2CrO4��ש��ɫ�����������Ե����һ��AgNO3��Һʱ������ש��ɫ������
�ʴ�Ϊ��AgCl�������һ��AgNO3��Һʱ������ש��ɫ������
����Һ��c��Ag+��=$\frac{2��1{0}^{-10}}{1{0}^{-5}}$=2��10-5mol/L����CrO42-Ũ��Ϊ$\frac{1.12��1{0}^{-12}}{��2��1{0}^{-5}��^{2}}$=2.8��10-3mol/L���ʴ�Ϊ��2.8��10-3��
����Һ�д���2CrO42-+2H+ Cr2O72-+H2O��pHС��c��H+��Ũ�ȴ�ƽ�����ƣ�ʹ�ⶨ�����ȷ��
Cr2O72-+H2O��pHС��c��H+��Ũ�ȴ�ƽ�����ƣ�ʹ�ⶨ�����ȷ��
�ʴ�Ϊ��2CrO42-+2H+ Cr2O72-+H2O��pHС��c��H+��Ũ�ȴ�ƽ�����ƣ�ʹ�ⶨ�����ȷ��
Cr2O72-+H2O��pHС��c��H+��Ũ�ȴ�ƽ�����ƣ�ʹ�ⶨ�����ȷ��
��4���������������ӵõ�����������������缫����ʽΪ��2H++2e-=H2����Cr2O72-���������ӷ���������ԭ��Ӧ����Cr3+�����������ӣ�
�����ӷ���ʽΪ��Cr2O72-+6Fe2++14H+=6Fe3++2Cr3++7H2O��
Fe2+��2e-����$\frac{1}{6}$Cr2O72-
6mol n
��n=0.5mol��
�ʴ�Ϊ��2H++2e-=H2����Cr2O72-+6Fe2++14H+=6Fe3++2Cr3++7H2O��0.5��
����ͼ��֪��pHԽС��Cr2O72-��ȥ����Խ�����γ�Cr��OH��3�����ӷ�ˮ�з��룻pHԽ��Cr2O72-��ȥ����ԽС��������Һ��pHȡֵ��4.3��5.6��Χ�ڶԽ��ͷ�ˮ�еĸ�������������
�ʴ�Ϊ��4.3��5.6��
���� ���⿼�����ܶȻ��������йؼ��㡢�ζ�ԭ����Ӧ�á���Һ�ij��ӡ�������ԭ��Ӧ�����ԭ����Ӧ�õȣ���Ŀ�Ѷ��еȣ������ڻ���֪ʶ���ۺ�Ӧ�õĿ��飬ע�����Ksp���йؼ��㷽����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | A��ԭ�ӵ�����������Bԭ�ӵ����������� | |
| B�� | Aԭ�ӵ���������Bԭ�ӵ����������� | |
| C�� | 1mol A���ᷴӦ�û�����H2��1mol B���ᷴӦ�û�����H2�� | |
| D�� | ��AB�õ������Ӻ����ʢ��ϡ������ձ��У�B�ϲ������� |
| A�� | O2��I2��Hg | B�� | CO2��KCl��SiO2 | ||
| C�� | HF��HCl��HBr | D�� | CH4��C2H5OH��C4H10 |
| A�� | ��������H2 C2O4���壬�ٽ�ˮ���룬��Һ��c��H+������ | |
| B�� | ����NaOH��Һ��ǡ����ȫ��Ӧ������Һ�У�c��Na+����c��C2O42-����c��HC2O4-����c��H+�� | |
| C�� | ���백ˮ�����ԣ�����Һ�У�c��NH4+��+c��Na+��=2c��C2O42-��+c��HC2O4-�� | |
| D�� | ����0.01 mol Na2C2O4���壬����Һ�У�3c��Na+��=2[c��H2C2O4��+c��HC2O4- ��+c��C2O42-��] |
��NH4I��s��?NH3��g��+HI��g��
��2HI��g��?H2��g��+I2��g��
5min���ƽ��ʱ��c��H2��=0.5mol/L��c��HI��=4mol/L��������˵������ȷ���ǣ�������
| A�� | ��NH3��ʾ��Ӧ�ٵ�����Ϊ1 mol/��L•min�� | |
| B�� | ��ϵ����ɫ���ٱ仯ʱ�����жϷ�Ӧ���Ѵ�ƽ�� | |
| C�� | ���¶��·�Ӧ�ٵ�ƽ�ⳣ��Ϊ20 mol2/L2 | |
| D�� | ��ѹʱ��Ӧ�ڵ�ƽ�ⲻ���ƶ� |
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� |
��2������ЩԪ���У���ѧ��������õ��ǣ�Ar����Ԫ�ط��ţ���
��3��������������ˮ�����У�������ǿ�Ļ�����ķ���ʽ��HClO4��������ǿ�Ļ�����ĵ���ʽ�ǣ�
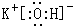 ��
����4������������������������Ԫ����Al����Ԫ�ط��ţ���д���������������������Ʒ�Ӧ�����ӷ���ʽAl2O3+2OH-=2AlO2-+H2O��
��5���õ���ʽ��ʾԪ�آ���Ļ�������γɹ��̣�
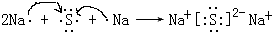 ���û������������ӣ�����ۡ������ӡ��������
���û������������ӣ�����ۡ������ӡ����������6����ʾ����۵Ļ�����ĵ���ʽ
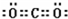 ���û��������ɼ��ԣ�����ԡ����Ǽ��ԡ������γɵģ�
���û��������ɼ��ԣ�����ԡ����Ǽ��ԡ������γɵģ� | A�� | �������ȷ�Ӧ���Ӹֹ죺2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$ 2Fe+Al2O3 | |
| B�� | ����ˮ��ȥFeCl3��Һ�е�Fe2+��Cl2+Fe2+�T2Cl-+Fe3+ | |
| C�� | ����֪Ũ�ȵ�NaOH��Һ�ⶨδ֪Ũ�ȵĴ�����Һ��Ũ�ȣ�H++OH-�TH2O | |
| D�� | ʢ��NaOH��Һ���Լ�ƿ�����ò�������SiO2+2Na++2OH-�TNa2SiO3+H2O |
| A�� | ��Һ�д���CO32- | B�� | ��Һ��c��Na+����c��CO32-�� | ||
| C�� | ������ʵ�����NaOHǡ���к� | D�� | ��Һ��c��H+��•c��OH-��=10-14 |
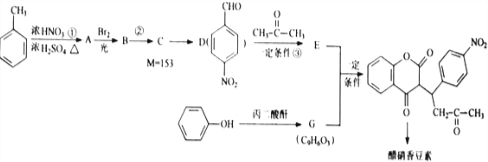
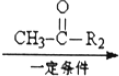 R1-CH=
R1-CH=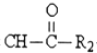 +H2O
+H2O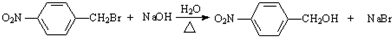 ��
��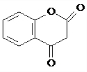 ��
��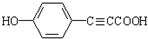 ��
��