��Ŀ����
11���±���Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ���д���пհף�| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� |
��2������ЩԪ���У���ѧ��������õ��ǣ�Ar����Ԫ�ط��ţ���
��3��������������ˮ�����У�������ǿ�Ļ�����ķ���ʽ��HClO4��������ǿ�Ļ�����ĵ���ʽ�ǣ�
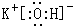 ��
����4������������������������Ԫ����Al����Ԫ�ط��ţ���д���������������������Ʒ�Ӧ�����ӷ���ʽAl2O3+2OH-=2AlO2-+H2O��
��5���õ���ʽ��ʾԪ�آ���Ļ�������γɹ��̣�
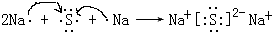 ���û������������ӣ�����ۡ������ӡ��������
���û������������ӣ�����ۡ������ӡ����������6����ʾ����۵Ļ�����ĵ���ʽ
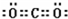 ���û��������ɼ��ԣ�����ԡ����Ǽ��ԡ������γɵģ�
���û��������ɼ��ԣ�����ԡ����Ǽ��ԡ������γɵģ�
���� ��1����Ԫ�������ڱ���λ�ÿ�֪���١�����Ԫ�طֱ�ΪC��N��O��Na��Al��S��Cl��Ar��K��
��2������Ԫ���У�ֻ��Ar�����������8�����ȶ��ṹ��
��3���ǽ�����Խǿ������������ˮ��������Խǿ��������Խǿ������������ˮ���������ǿ��
��4��������Ϊ�����������NaOH��Ӧ����ƫ�����ƺ�ˮ��
��5������Ļ�����Ϊ���ƣ��������Ӻ�������֮��ͨ�����Ӽ��γɵ����ӻ����
��6������۵Ļ�����Ϊ������̼����̼ԭ�Ӻ���ԭ�Ӽ�ͨ�����Թ��ۼ��γɵĹ��ۻ����
��� �⣺��1����Ԫ�������ڱ���λ�ÿ�֪���١�����Ԫ�طֱ�ΪC��N��O��Na��Al��S��Cl��Ar��K���ʴ�Ϊ��C��N��O��Na��Al��S��Cl��Ar��K��
��2������Ԫ���У�ֻ��Ar�����������8�����ȶ��ṹ����ѧ�������ȶ�����ϡ������Ar���ʴ�Ϊ��Ar��
��3������Ԫ����ClԪ�صķǽ�������ǿ��������ǿ�Ļ�����ķ���ʽ��HClO4��K�Ľ�������ǿ����Ӧ�ļ�ΪKOH��������ǿ��Ϊ���ӻ���������ʽΪ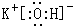 ���ʴ�Ϊ��HClO4��
���ʴ�Ϊ��HClO4��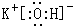 ��
��
��4��AlԪ�ض�Ӧ��������Ϊ�����������NaOH��Ӧ����ƫ�����ƺ�ˮ�����ӷ�ӦΪAl2O3+2OH-=2AlO2-+H2O��
�ʴ�Ϊ��Al��Al2O3+2OH-=2AlO2-+H2O��
��5������Ļ�����Ϊ���ƣ���ͨ�����Ӽ��γɵ����ӻ�����õ���ʽ��ʾ�γɹ���Ϊ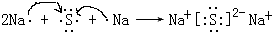 ��
��
�ʴ�Ϊ��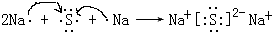 �����ӣ�
�����ӣ�
��6������۵Ļ�����Ϊ������̼����̼ԭ�Ӻ���ԭ�Ӽ�ͨ�����Թ��ۼ��γɵĹ��ۻ���������ʽΪ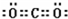 ���ʴ�Ϊ��
���ʴ�Ϊ��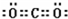 �����ԣ�
�����ԣ�
���� ���⿼��λ�á��ṹ�����ʵĹ�ϵ��Ӧ�ã�Ϊ��Ƶ���㣬�漰Ԫ�ص�λ�á�Ԫ�����ڱ���Ԫ�������ɵĹ�ϵ�ȣ���Ŀ�ѶȲ���ע�����ʽ����д�Լ��õ���ʽ��ʾ�γɹ��̵IJ�ͬ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | 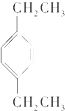 | B�� | 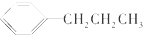 | C�� | 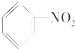 | D�� |  |
| A�� | �Ҷ���������������Һ | |
| B�� | ��ѿ����ˮ��ǰ����ܷ���������Ӧ | |
| C�� | 1-�ȱ����2-�ȱ��鷢����ȥ��Ӧ�IJ��ﲻͬ | |
| D�� | H2N-CH2-COOH�����۲����к�  �ṹ �ṹ |
��֪K2Cr2O7��Һ�д���ƽ��Cr2O72-+H2O?2CrO42-+2H+��
�ش��������⣨��������Һ�е�����Ũ��С��1��10-5 mol•L-1ʱ����������Ϊ������ȫ����
��1���ظ�����Լ���ǩ��Ӧ��ע��ͼ1��A��������ĸ��ţ�
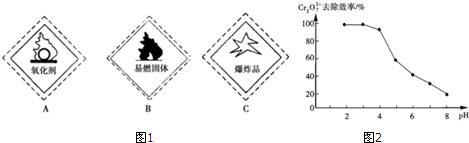
��2������ʱ���ظ������Ũ���ᷴӦ��ʹ�����������ݳ�����д���÷�Ӧ�����ӷ���ʽ��14H++Cr2O72-+6Cl-$\frac{\underline{\;\;��\;\;}}{\;}$2Cr3++3Cl2��+7H2O��
��3����֪AgCl��Ag2CrO4��ש��ɫ����Ksp�ֱ�Ϊ2��10-10��1.12��10-12��������ѧ�У��ⶨ���ȵ�������Һ��Cl-�ĺ�������K2CrO4��ָʾ������AgNO3��Һ�ζ����ζ�������������������AgCl���ﵽ�ζ��յ��ʵ������Ϊ�������һ��AgNO3��Һʱ������ש��ɫ�������ó����ζ���Ҫע���������⣺
��ָʾ�����������ζ��յ�ʱ����Һ�е�CrO42-Ũ��Ϊ2.8��10-3 mol•L-1ʱ���ʣ�
�ڿ�����Һ����ȣ�pH��6.5-10.5֮�䣬���Ϸ�Ӧ����ʽ������ΪʲôpH��6.5ʵ��������ȷ��2CrO42-+2H+
 Cr2O72-+H2O��pHС��c��H+��Ũ�ȴ�ƽ�����ƣ�ʹ�ⶨ�����ȷ��
Cr2O72-+H2O��pHС��c��H+��Ũ�ȴ�ƽ�����ƣ�ʹ�ⶨ�����ȷ����4�������ڸ�Ԫ�صĺ������ߣ����������ж��������°�����ҵ�Ͽɲ�������������⺬Cr2O72-�����Է�ˮ�����ŵ��Ľ��У�������������ҺpH���ߣ�����Fe��OH��3��Cr��OH��3�������Ӷ�ʹ��ˮ�и����������ŷű����±��dz����½������������Ksp�ͽ���������ijŨ���¿�ʼ���������pH������Ũ��Ϊ��ӦpHʱ��Һ���йؽ������Ӳ�����������СŨ�ȣ�[Cr��OH��3��һ��������������]��
| �������� | Ksp | pH��10-1 mol•L-1�� | pH��10-5 mol•L-1�� |
| Fe3+ | 4.0��10-38 | 2.7 | 3.7 |
| Cr3+ | 6.0��10-31 | 4.3 | 5.6 |
��pH�Է�ˮ��Cr2O72-ȥ��Ч�ʵ�Ӱ�������ͼ2��ʾ���������жԽ��ͷ�ˮ�еĸ�������������pH��ΧΪ4.3��5.6��
| A�� | �ɵ�� | B�� | ﮵�� | C�� | ̫���ܵ�� | D�� | Ǧ���� |
| A�� | ��ͭƬ��Ǧ��о���缫����ϡ������ | |
| B�� | ������ͭƬ���缫������������Һ�� | |
| C�� | ��пƬ��ͭƬ���缫���뷬���� | |
| D�� | ��ͭƬ����Ƭ���缫����ƾ��� |
| A�� | SO32-����ԭ�ӵ��ӻ���ʽΪsp3 | B�� | H2O��������ԭ�ӵ��ӻ���ʽΪsp2 | ||
| C�� | BF3���ӳ�������ռ��� | D�� | C2H2�����к���3���Ҽ���2���м� |
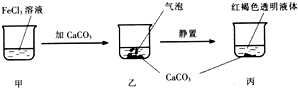
| A�� | �������������C02 | |
| B�� | ����Һ��ɲ������������ | |
| C�� | ��������Һ����ı仯���ձ���c��Cl-���������仯 | |
| D�� | ����CaC03����CaS04Ҳ�ɵõ���ͬ��ʵ������ |
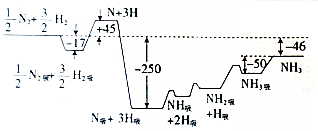 ��ѧ��Ӧԭ���ڹ�ҵ�����о���ʮ����Ҫ�����壮
��ѧ��Ӧԭ���ڹ�ҵ�����о���ʮ����Ҫ�����壮