��Ŀ����
16�� Ŀǰ��ȫ��������������������ͭ������Ǧ��п������ɫ��������λ������ҵ��չ�̲��ž�DZ����
Ŀǰ��ȫ��������������������ͭ������Ǧ��п������ɫ��������λ������ҵ��չ�̲��ž�DZ������1�������Ni��CO��4������ΪҺ̬��������CCl4�������л��ܼ�����̬Ni��CO��4���ڷ��Ӿ��壻NiԪ����Ԫ�����ڱ��е�λ���ǵ��� ���ڣ��ڢ� �壻����${\;}_{28}^{63}$Ti��${\;}_{28}^{57}$Ti����ԭ�ӣ����ǻ���Ϊͬλ�أ�
��2����ͼ��ʾ���������ڵ���������ACE�����ţ���
A����� B�����Ӽ� C�����ۼ� D�������� E����λ��
��3���ܶ�����л�����Ni���¿�����H2�����ӳɷ�Ӧ���� ��CH2=CH2����CH��CH����
 ����HCHO�ȣ����з�������ƽ��ṹ���Т٢ڢۢܣ���������ţ���Ԥ��HCHO���ӵ�����ṹΪƽ�������Σ�
����HCHO�ȣ����з�������ƽ��ṹ���Т٢ڢۢܣ���������ţ���Ԥ��HCHO���ӵ�����ṹΪƽ�������Σ���4�����������������Դ���磨La��������Ni���ĺϽ����������ϣ��úϽ�ľ�����ͼ��ʾ
 ������������һ����ԭ�ӣ�������ԭ�Ӷ��ھ������ϣ���ԭ�Ӷ��ھ��������ϣ��þ���Ļ�ѧʽΪNi5La��LaNi5��
������������һ����ԭ�ӣ�������ԭ�Ӷ��ھ������ϣ���ԭ�Ӷ��ھ��������ϣ��þ���Ļ�ѧʽΪNi5La��LaNi5��
���� ��1������Ni��CO��4���۵㡢�ܽ����жϾ������ͣ�NiԪ����28��Ԫ�أ����ݺ�������Ų�������д��̬ԭ�ӵĵ����Ų�ʽ��ȷ�������ڱ��е�λ�ã���������ͬ����������ͬ��ԭ�ӻ�Ϊͬλ�أ�
��2������ͼ��֪̼̼����̼����Ϊ���ۼ���������Ϊ��λ�����������ڹ��ۼ��������
��3��CH2=CH2�� Ϊƽ���ͽṹ����ȲΪֱ���ͽṹ������HCHO��Cԭ�Ӽ۲���Ӷ�����µ��Ӷԣ��ж�HCHO����ṹ��
Ϊƽ���ͽṹ����ȲΪֱ���ͽṹ������HCHO��Cԭ�Ӽ۲���Ӷ�����µ��Ӷԣ��ж�HCHO����ṹ��
��4�����þ�̯�����㾧����Ni��Laԭ����Ŀ������ȷ���仯ѧʽ��
��� �⣺��1�������Ni��CO��4����ΪҺ̬���۵�ͣ�������CCl4�������л��ܼ�������Ni��CO��4���ڷ��Ӿ��壻NiԪ����28��Ԫ�أ�λ�ڵ������ڵڢ��壬���̬ԭ�ӵĵ����Ų�ʽ1s22s22p63s23p63d84s2�����ڵ������ڢ��壻6328Ti��5728Ti����������ͬ����������ͬ��ԭ�ӣ�����Ϊͬλ�أ�
�ʴ�Ϊ�����ӣ��ġ�����ͬλ�أ�
��2������ͼ��֪̼̼����̼����Ϊ���ۼ���������Ϊ��λ�����������ڹ��ۼ��������
��ѡ��ACE��
��3��CH2=CH2�� Ϊƽ���ͽṹ����ȲΪֱ���ͽṹ��һ��Ϊƽ��ṹ��HCHO��̼ԭ�Ӽ۲���Ӷ���Ϊ3+$\frac{4-1-1-2}{2}$=3��û�й¶Ե��ӣ�����ռ乹��Ϊƽ�������Σ�Ҳ����ƽ��ṹ��
Ϊƽ���ͽṹ����ȲΪֱ���ͽṹ��һ��Ϊƽ��ṹ��HCHO��̼ԭ�Ӽ۲���Ӷ���Ϊ3+$\frac{4-1-1-2}{2}$=3��û�й¶Ե��ӣ�����ռ乹��Ϊƽ�������Σ�Ҳ����ƽ��ṹ��
�ʴ�Ϊ���٢ڢۢܣ�ƽ�����ǣ�
��4������������һ����ԭ�ӣ�����8����ԭ�Ӷ��ھ������ϣ���ԭ�Ӷ��ھ������㣮���Ծ����к��е���ԭ��Ϊ1+8��$\frac{1}{2}$=5���������е���ԭ��Ϊ8��$\frac{1}{8}$=1�����Ծ���Ļ�ѧʽNi5La��LaNi5��
�ʴ�Ϊ��Ni5La��LaNi5��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰�������������ʡ���ѧ�������ӿռ�ṹ����������ȣ��Ѷ��еȣ�ע�����þ�̯�����о������йؼ��㣮

 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�| A�� | �ԣ�A��=0.20mol/��L•min�� | B�� | �ԣ�B��=0.45 mol/��L•min�� | ||
| C�� | �ԣ�C��=0.30 mol/��L•min�� | D�� | �ԣ�D��=0.35 mol/��L•min�� |
| A�� | ������֧�Թܵ���ɫ������ͬ | |
| B�� | A�Թ���Fe��OH��3������� | |
| C�� | ֱ�Ӽ�������Fe2��SO4��3��Һ�ɵõ�Fe2��SO4��3���� | |
| D�� | B�Թ���Fe2��SO4��3���ܷ���ˮ�� |
| A�� | ͭ��ʯ��ϸ��������ֱ��ת��Ϊ����ͭ����������н���������ͭ | |
| B�� | ����ͨ���ñ�ȼ���Ȼ���ֵ������ȼ��ȼ�շų������Ĵ�С����ͬ�����£�ij���ʵ���ֵԽ�ߣ����ȼ��Խ�� | |
| C�� | Ǧ����������Ķ��ε�أ��ɸ���������ܶ����ж�Ǧ�����Ƿ���Ҫ��� | |
| D�� | ���ع��͡���ֹʳ�ã�������ͨ�������仯�Ʒ��� |
 ��֪�ϳɰ���ӦN2��g��+3H2��g��?2NH3��g����H=-92.2kJ/mol��
��֪�ϳɰ���ӦN2��g��+3H2��g��?2NH3��g����H=-92.2kJ/mol����1���÷�Ӧ�Ļ�ѧƽ�ⳣ��K���¶�T�Ĺ�ϵ����
| T/K | 473 | 573 | 673 | �� |
| K | 4.4��10-2 | K1 | K2 | �� |
��2���ϳɰ���ũҵ�������к���Ҫ�����壬��ʵ�������У����������д�ʩ�����п�������������ԭ�����͵���BD
A��������ý�ӿ컯ѧ��Ӧ����
B�����ýϸ�ѹǿ��20MPa��50MPa ��
C�����ýϸ��¶� ��400�桫500�棩
D�������ɵİ�Һ������ʱ����ϵ�з������
��3����ijһ�¶��£����������������м���2molN2��8molH2�����������ƽ��ʱ������������ѹǿΪ��ʼ��80%����Ӧ�ﵽƽ��ʱ��N2��ת������50%����ʱ��Ӧ�ų�������=92.2kJ���������������=����
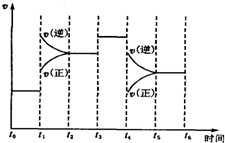
��4����ij��ʱ��t0��t6�з�Ӧ������ʱ�������ͼ���£��ٷֺ�����ߵ�ʱ���A
A��t0��t1 B��t2��t3 C��t3��t4 D��t5��t6
��5��t1ʱ�̸ı�������ǣ������¶ȣ�t3ʱ�̸ı�������ǣ����������t4ʱ�̸ı�������Ǽ�Сѹǿ��

| A�� | �������������������Mg��Ӧ��HX�ų�����������С | |
| B�� | ������������Ϻ���NaOH��Һ��Ӧ����HXǡ����ȫ��Ӧʱ��c��Y-����c��X-����c��OH-����c��H+�� | |
| C�� | �������ߣ��ɵ�0.1mol•L-1HX��Һ����ˮ�������c��H+��Ϊ10-4mol•L-1 | |
| D�� | ��HY��HZ��ϡ��Һ��Ϻ�������Һ�У�c��H+��=$\frac{{K}_{a}��HY��•c��HY��}{c��{H}^{+}��}$+c��Z-��+c��OH-�� |
| A�� |  �ص�ȼ��ʵ�� | B�� |  ��ǿ��ζ�ǿ�� | ||
| C�� |  ����H2O��g����Ӧ | D�� |  ̽���¶ȶ�ƽ���ƶ���Ӱ�� |
| A�� | �ⶨij��Ԫ����������Һ��pH������С��7 | |
| B�� | ϡ��0.1mol/LCH3COOH��aq����$\frac{c��O{H}^{-}��c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}$���ܱ��Ҳ���ܱ�С | |
| C�� | ��20.00mlŨ�Ⱦ�Ϊ0.100 mol/L��HCl��CH3COOH�����Һ�еμ�0.200mol/LNaOH��Һ��pH=7��NaOH��Һ���һ��С��20.00ml | |
| D�� | pH=8�ļ�����Һ��һ��û��CH3COOH���� |
 ��
��