��Ŀ����
3����ѧ�����о�������������ֵĹ����У�������Cu-Ni-Fe�ȶ��ֽ����������1������ij�ֽ����������Ǿ��廹�ǷǾ���ķ�����X�������䣮
��2����֪������Cu2O��CuO���ȶ����Դ�ͭԭ�Ӻ�����ӱ仯�ǶȽ�����ԭ��Cu+�����������Ų�ʽΪ3d10����Cu2+�����������Ų�Ϊ3d9����Ϊ���������Ų��ﵽȫ��ʱ�ȶ������Թ�̬Cu2O�ȶ���ǿ��CuO��
��3����������±�أ�SCN��2��Ӧ����Ni��SCN��2����SCN��2��������ԭ�ӵ��ӻ���ʽ��sp3���Ҽ��ͦм���Ŀ֮��Ϊ5��4����±�أ�SCN��2��Ӧ���������֣������������ᣨH-S-C��N���ķе�����������ᣨH-N�TC�TS���ķе㣬��ԭ��������������Ӽ����γ��������������γ������
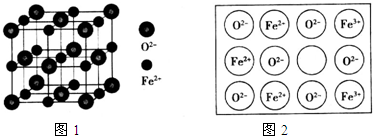
��4������FeO����Ľṹ��ͼ1��ʾ���辧���߳�Ϊa cm���ܶ�Ϊb g•cm-3�����ӵ������ɱ�ʾΪ$\frac{288}{{a}^{3}b}$/mol���ú�a��b��ʽ�ӱ�ʾ�����˹��Ʊ���FeO���峣����ȱ�ݣ���ͼ2������֪ij��������Ʒ���ΪFe0.96O���þ�����Fe3+��Fe2+�����Ӹ���֮��Ϊ1��11��
���� ��1�����塢�Ǿ���ķ�����X�������䣻
��2������е��Ӵ���ȫ����ȫ�ա�����ʱԭ�����ȶ���
��3����SCN��2�����з��ӽṹʽΪN��C-S-S-C��N��ÿ��Sԭ�Ӽ۲���ӶԸ�����4�Һ��������µ��Ӷԣ����ݼ۲���ӶԻ��������ж���ԭ�ӵ��ӻ���ʽ���÷����ЦҼ��ͦм���Ŀ֮��Ϊ5��4����������������۷е�ϸߣ�
��4���þ������������Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4�������Ӹ���=1+12��$\frac{1}{4}$=4��
�辧���߳�Ϊa cm���������Ϊa3cm3���ܶ�Ϊb g•cm-3�������ӵ�����=$\frac{4M}{��V}$��
ij��������Ʒ���ΪFe0.96O���������и�Ԫ�ػ��ϼ۵Ĵ�����Ϊ0�����ݵ���غ��жϸþ�����Fe3+��Fe2+�����Ӹ���֮�ȣ�
��� �⣺��1�����塢�Ǿ���ķ�����X�������䣬�ʴ�Ϊ��x�������䣻
��2������е��Ӵ���ȫ����ȫ�ա�����ʱԭ�����ȶ���Cu+�����������Ų�ʽΪ3d10����Cu2+�����������Ų�Ϊ3d9����Ϊ���������Ų��ﵽȫ��ʱ�ȶ������Թ�̬Cu2O�ȶ���ǿ��CuO���ʴ�Ϊ��Cu+�����������Ų�ʽΪ3d10����Cu2+�����������Ų�Ϊ3d9����Ϊ���������Ų��ﵽȫ��ʱ�ȶ������Թ�̬Cu2O�ȶ���ǿ��CuO��
��3����SCN��2�����з��ӽṹʽΪN��C-S-S-C��N��ÿ��Sԭ�Ӽ۲���ӶԸ�����4�Һ��������µ��Ӷԣ����ݼ۲���ӶԻ�������֪��ԭ�ӵ��ӻ���ʽΪsp3���÷����ЦҼ��ͦм���Ŀ֮��Ϊ5��4����������������۷е�ϸߣ�����������Ӽ����γ��������������γ���������������ᣨH-S-C��N���ķе�����������ᣨH-N�TC�TS���ķе㣬
�ʴ�Ϊ��sp3��5��4������������Ӽ����γ��������������γ������
��4���þ������������Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4�������Ӹ���=1+12��$\frac{1}{4}$=4��
�辧���߳�Ϊa cm���������Ϊa3cm3���ܶ�Ϊb g•cm-3�������ӵ�����=$\frac{4M}{��V}$=$\frac{4��72}{b��{a}^{3}}$/mol=$\frac{288}{{a}^{3}b}$/mol��
ij��������Ʒ���ΪFe0.96O���������и�Ԫ�ػ��ϼ۵Ĵ�����Ϊ0�����ݵ���غ��жϸþ�����Fe3+��Fe2+�����Ӹ���֮�ȣ���Fe3+��Fe2+�ĸ����ֱ���x��y��$\left\{\begin{array}{l}{x+y=0.96}\\{3x+2y=2}\end{array}\right.$����
$\left\{\begin{array}{l}{x=0.08}\\{y=0.88}\end{array}\right.$������x��y=0.08��0.88=1��11��
�ʴ�Ϊ��$\frac{288}{{a}^{3}b}$/mol��1��11��
���� ���⿼�����ʽṹ�����ʣ�Ϊ��Ƶ���㣬�漰�������㡢ԭ���ӻ���ʽ�жϡ������ԭ�Ӻ�������Ų���֪ʶ�㣬֪���������������Ӱ�졢����ԭ�����۲���ӶԻ������ۡ���̯����֪ʶ�㼴�ɽ���ѵ��ǣ�4���⾧�����㣬��Ŀ�Ѷ��еȣ�

 53���ò�ϵ�д�
53���ò�ϵ�д�| A�� | 25��ʱ��pH=1�Ĵ�����Һ�к���H+����ĿΪ0.1NA | |
| B�� | 100mL 18.4mol•L-1 ��Ũ������������ͭ���ȷ�Ӧ������SO2 ������Ϊ0.92NA | |
| C�� | ��CO2 ͨ��Na2O2 ����������������a gʱ����Ӧ��ת�Ƶĵ�����Ϊa$\frac{{N}_{A}}{28}$ | |
| D�� | ��״���£�11.2L�������������õ��Ӷ���Ϊ7NA |
��������衿
����1������ΪCu ��OH��2
����2������ΪCuCO3
����3������Ϊ��ʽ̼��ͭ[��ѧʽ�ɱ�ʾΪnCuCO3•mCu��OH��2]
���������ϡ���������һ�ֳ������Ⱦ��ֽ⣨����������ᾧˮ����
������̽����
����1������������Һ���ˣ�������ˮϴ�ӣ�������ˮ�Ҵ�ϴ�ӣ���ɣ�
����2����ͬѧȡһ�������壬�����������õ�����װ�ã��г�����δ���������ж���ʵ�飻
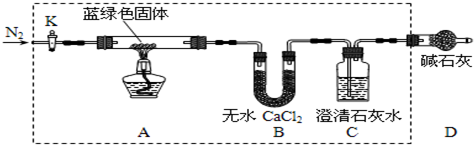
��1������Ӧ��A������ɫ�����ڣ�C������������֤������1������
��2����ͬѧ��ΪֻҪ����ͼ��Bװ�õ��Լ���������ij�Լ������֤�������м��裬���Լ���B������ţ���
A��Ũ���� B����ˮCuSO4 C����ʯ�� D��P2O5
��3����ͬѧ��֤����3������ʵ��������A������ɫ������ɫ��B����ˮCuSO4���������C���а�ɫ����������
������̽����
��4����ͬѧ��һ��̽������3�й������ɣ�
����ͬѧ���һЩ������20������ݣ��������C�еij���ʯ��ˮ��ΪBa��OH��2��Һ����ԭ����AC������ţ�
| �ܽ�ȣ�S��/g | �ܶȻ���Ksp�� | Ħ��������M��/g•mol-1 | |||
| Ca��OH��2 | Ba��OH��2 | CaCO3 | BaCO3 | CaCO3 | BaCO3 |
| 0.16 | 3.89 | 2.9��10-9 | 2.6��10-9 | 100 | 197 |
B��Ba��OH��2Ϊǿ�Ca��OH��2Ϊ����
C�����յ���CO2���ɵ�BaCO3����������CaCO3���������С
D����ͬ�����£�CaCO3���ܽ�����Դ���BaCO3
������ȡ����ɫ��������Ϊ54.2g��ʵ�������װ��B����������5.4g��C�еIJ�������������Ϊ39.4g���������ɫ����Ļ�ѧʽΪ2CuCO3•3Cu��OH��2��A�з����ķ�Ӧ�Ļ�ѧ����ʽΪ2CuCO3•3Cu��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$5CuO+3H2O��+2CO2����
| A�� | x=y | B�� | x��y | C�� | x��y | D�� | ��ȷ�� |
 ��ˮ������EΪ�������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�
��ˮ������EΪ�������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�
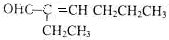 ����ֻ��һ��ȡ�������������C��2�ֹ����ŵļ�Ҫ��������������Һ�ȼ���ȩ�����ټ�ϡ����ʹ��Һ�����Ժ���ˮ����̼̼˫����
����ֻ��һ��ȡ�������������C��2�ֹ����ŵļ�Ҫ��������������Һ�ȼ���ȩ�����ټ�ϡ����ʹ��Һ�����Ժ���ˮ����̼̼˫���� ��HOCH2CH2C��C-C��C-COOH��
��HOCH2CH2C��C-C��C-COOH�� ��
�� ��
��
 ��
��