��Ŀ����
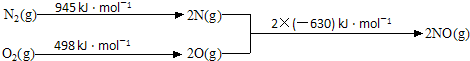
14��1967��������������������ĸ���䶨��Ϊ��������仯����ӵ�����ֻ��������ϵĽ���Ԫ�أ���Cu9Al4��Cu5Zn8�ȣ��ش��������⣺
��1��ij�ֽ�������������Է��ԣ�ԭ������ά�ռ�����������������У��ý������������ھ��� ������塱�Ǿ��塱����
��2����̬ͭԭ����1��δ�ɶԵ��ӣ�����ͭ���ӵĵ����Ų�ʽΪ1s22s22p63s23p63d9����CuS04��Һ�е��������ˮ���γ�����ɫ��Һ�����ʵĻ�ѧʽΪ[Cu��NH3��4]S04��
��3��ͭ������±�أ�SCN��2��Ӧ����Cu��SCN��2��1mol��SCN��2�����к��Цм�����ĿΪ5NA����±�أ�SCN��2��Ӧ���������֣��������������ᣨH-S-C��N�����������ᣨH-N�TC�TS�������зе�ϸߵ�������������Ӽ���γ��������������ܣ��ѧ���ƣ�
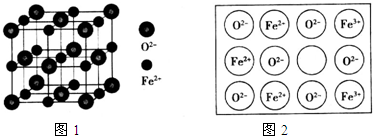
��4��ZnS�ľ����ṹ��ͼ1��ʾ����ZnS�����У�S2-����λ��Ϊ4��
��5��ͭ����γɵĽ���������ṹ��ͼ2���侧���߳�Ϊa nm���ý�����������ܶ�Ϊ$\frac{389}{{N}_{A}•{a}^{3}��1{0}^{-21}}$���ú���a��NA �Ĵ���ʽ��ʾ��g•cm-3��
���� ��1�����ݾ���ͷǾ��������������ص������
��2��CuΪ29��Ԫ�أ������Ų�ʽΪ1s22s22p63s23p63d104s1��ͭ�����백���γ��������[Cu��NH3��4]2+��
��3����SCN��2���ӽṹʽΪN��C-S-S-C��N��1��N��C������1���ȼ�����������Ϊ�м������Ӽ����������ʹ�����۷е����ߣ�
��4�����ݾ����Ľṹͼ����S2-����λ����
��5�����ݾ�̯�������������Cu��Auԭ���������ݦ�=$\frac{m}{V}$�����ܶȣ�
��� �⣺��1����������������ά�ռ�����������������У����Է��ԣ����Ǿ�����ԭ����������������Է��ԣ�
�ʴ�Ϊ�����壻
��2��CuΪ29��Ԫ�أ������Ų�ʽΪ1s22s22p63s23p63d104s1�����Կ���ֻ��4S����ϵĵ���δ�ɶԣ�����ͭ���ӵĵ����Ų�ʽΪ1s22s22p63s23p63d9����CuS04��Һ �е��������ˮ���γ������Ϊ[Cu��NH3��4]2+��������ɫ��Һ�����ʵĻ�ѧʽΪ[Cu��NH3��4]S04��
�ʴ�Ϊ��1��1s22s22p63s23p63d9��[Cu��NH3��4]S04��
��3����SCN��2���ӽṹʽΪN��C-S-S-C��N��1��N��C������1���ȼ�����������Ϊ�м���1mol��SCN��2�����к��Цȼ�����ĿΪ5NA���������ᣨH-N=C=S��������Nԭ����������Hԭ�ӣ����Ӽ����γ�������ʷе�ߣ�
�ʴ�Ϊ��5NA������������Ӽ���γ��������������ܣ�
��4������ͼ1������S2-�����п������4������S2-����λ��Ϊ4��
�ʴ�Ϊ��4��
��5��Cuԭ��λ�ھ������ģ���ĿΪ6��$\frac{1}{2}$=3��Auԭ��Ϊ�������㣬��ĿΪ8��$\frac{1}{8}$=1���������V=��a��10-7��3��
���ܶȦ�=$\frac{m}{V}$=$\frac{\frac{64��3+197}{{N}_{A}}}{{a}^{3}��1{0}^{-21}}$=$\frac{389}{{N}_{A}•{a}^{3}��1{0}^{-21}}$g•cm-3��
�ʴ�Ϊ��$\frac{389}{{N}_{A}•{a}^{3}��1{0}^{-21}}$��
���� ���⿼���˾���ṹ����������Ų���������Լ������ļ����֪ʶ�����ڿ���ѧϰ��֪�����Ϣ�������ͽ��������������Ѷ��еȣ�

| A�� | ��ȡ��̼���ڼ�����ʽ | |
| B�� | ���չ涨�������������з������ | |
| C�� | ����ũ���������������֪ʶ | |
| D�� | ����ʹ��һ���Կ��ӡ�ֽ�������ϴ��� |
| A�� | c��A-����c��B+ ����c��H+����c��OH-�� | B�� | c��B+ ��+c��H+ ��=c��A-��+c��OH-�� | ||
| C�� | c��B+ ����c��A-����c��H+ ��=c��OH-�� | D�� | c��BOH��+c��OH-��=c��H+�� |
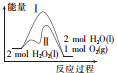
| A�� | ;������;������ȣ������Ǽ����˶������� | |
| B�� | 2 molH2O2��l������������2 molH2O��l�������� | |
| C�� | ����������ͬ��������ͬ������ʱ�ų���������;�������;���� | |
| D�� | ����������ͬ��������ͬ��������ʱ��;�������;���� |
| A�� | ���ܽ�����ݣ����ж����Mg��HCO3��2��Һ���ò�����Mg��OH��2����MgCO3 | |
| B�� | �÷е����ݣ����Ʋ��ܷ�һЩҺ�������÷���ķ������뿪���Ŀ����� | |
| C�� | �÷�Ӧ�����ݵĴ�С�����жϲ�ͬ��Ӧ�ķ�Ӧ���ʵĿ��� | |
| D�� | ��ԭ�Ӱ뾶���ݣ����ƶ�ijЩԭ�������Ի�ԭ�Ե�ǿ�� |
| A�� | ͨ��������������������Ҵ����������� | |
| B�� | ��ըʳ��Ļ����ͺ�ţ�Ͷ��ǿ������ı������� | |
| C�� | ���ۿ������Ʊ������ǡ�����ʳ�ף�������ҩƬ�ĸ��μ� | |
| D�� | ����̫�����ڴ��������·ֽ�ˮ�����ǰѹ���ת��Ϊ��ѧ�ܵ���ɫ��ѧ���� |