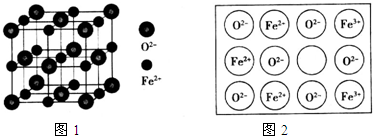
��Ŀ����
18��CuSO4��Һ��Na2CO3��Һ��ϲ�������ɫ������������ij��ȤС��Գ�����ɵ�̽������������衿
����1������ΪCu ��OH��2
����2������ΪCuCO3
����3������Ϊ��ʽ̼��ͭ[��ѧʽ�ɱ�ʾΪnCuCO3•mCu��OH��2]
���������ϡ���������һ�ֳ������Ⱦ��ֽ⣨����������ᾧˮ����
������̽����
����1������������Һ���ˣ�������ˮϴ�ӣ�������ˮ�Ҵ�ϴ�ӣ���ɣ�
����2����ͬѧȡһ�������壬�����������õ�����װ�ã��г�����δ���������ж���ʵ�飻
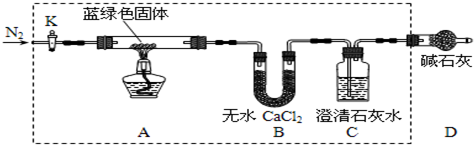
��1������Ӧ��A������ɫ�����ڣ�C������������֤������1������
��2����ͬѧ��ΪֻҪ����ͼ��Bװ�õ��Լ���������ij�Լ������֤�������м��裬���Լ���B������ţ���
A��Ũ���� B����ˮCuSO4 C����ʯ�� D��P2O5
��3����ͬѧ��֤����3������ʵ��������A������ɫ������ɫ��B����ˮCuSO4���������C���а�ɫ����������
������̽����
��4����ͬѧ��һ��̽������3�й������ɣ�
����ͬѧ���һЩ������20������ݣ��������C�еij���ʯ��ˮ��ΪBa��OH��2��Һ����ԭ����AC������ţ�
| �ܽ�ȣ�S��/g | �ܶȻ���Ksp�� | Ħ��������M��/g•mol-1 | |||
| Ca��OH��2 | Ba��OH��2 | CaCO3 | BaCO3 | CaCO3 | BaCO3 |
| 0.16 | 3.89 | 2.9��10-9 | 2.6��10-9 | 100 | 197 |
B��Ba��OH��2Ϊǿ�Ca��OH��2Ϊ����
C�����յ���CO2���ɵ�BaCO3����������CaCO3���������С
D����ͬ�����£�CaCO3���ܽ�����Դ���BaCO3
������ȡ����ɫ��������Ϊ54.2g��ʵ�������װ��B����������5.4g��C�еIJ�������������Ϊ39.4g���������ɫ����Ļ�ѧʽΪ2CuCO3•3Cu��OH��2��A�з����ķ�Ӧ�Ļ�ѧ����ʽΪ2CuCO3•3Cu��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$5CuO+3H2O��+2CO2����
���� ������裺������ͭ��̼��ͭ������ɫ������
��1������ʱ��̼��ͭ�ֽ����ɶ�����̼�ͺ�ɫ����ͭ��������̼��ʹ�����ʯ��ˮ����ǣ�����������ͭʱ����ˮ�����ͺ�ɫ����ͭ��ˮ��ʹ��ˮ����ͭ������
��2��ʵ����֤̼��ͭ��������ͭ���������ȷֽ����ɵIJ���������֤������ʱ��̼��ͭ�ֽ����ɶ�����̼�ͺ�ɫ����ͭ������������ͭʱ����ˮ�����ͺ�ɫ����ͭ��������Ҫ��֤ˮ�Ĵ��ںͶ�����̼�Ĵ��ھͿ���֤�����������ʳɷ֣�
��3�����ݣ�2���ķ�������ʵ������
��4��������ͼ�������ݷ����ж������ܽ�Ⱥ�������ʵ�����������С������
��B�����յ���ˮ��C�����յ��Ƕ�����̼�����������غ㶨�ɲ������ͭ������������ԭ���غ�ȷ���仯ѧʽ��CuCO3•3Cu��OH��2���ȷֽ�����CuO��������̼��ˮ��
��� �⣺CuSO4��Һ��Na2CO3��Һ��ϲ�������ɫ�����������Ƿ�Ӧ������̼��ͭ��������˫ˮ��������������ͭ���������Ǽ�ʽ̼��ͭ������1�г���ΪCu��OH��2������2ΪCuCO3��
�ʴ�Ϊ��CuCO3��
��1������ʱ��̼��ͭ�ֽ����ɶ�����̼�ͺ�ɫ����ͭ��������̼��ʹ�����ʯ��ˮ����ǣ�����������ͭʱ����ˮ�����ͺ�ɫ����ͭ������Ӧ��A������ɫ�����ڣ�C������������֤��һ��������̼��ͭ������IJ���Ϊ������ͭ������1��ȷ��
�ʴ�Ϊ��1��
��2��ʵ����֤̼��ͭ��������ͭ���������ȷֽ����ɵIJ���������֤������ʱ��̼��ͭ�ֽ����ɶ�����̼�ͺ�ɫ����ͭ������������ͭʱ����ˮ�����ͺ�ɫ����ͭ��������Ҫ��֤ˮ�Ĵ��ںͶ�����̼�Ĵ��ھͿ���֤�����������ʳɷ֣�װ��A�м����Ƿ�仯Ϊ��ɫ���壬װ��Bѡ����ˮ����ͭ��֤�Ƿ�����ˮ������ʯ��ˮ�Ƿ�����֤���Ƿ����ɶ�����̼��
�ʴ�Ϊ��B��
��3��װ��A�м����Ƿ�仯Ϊ��ɫ���壬װ��Bѡ����ˮ����ͭ���Ƿ����ɫ��֤�Ƿ�����ˮ��װ��C�г���ʯ��ˮ�Ƿ�����֤���Ƿ����ɶ�����̼������֤�������Ƿ���ȷ������Ϊ��A������ɫ������ɫ��B����ˮCuSO4���������C���а�ɫ����������
�ʴ�Ϊ��A������ɫ������ɫ��B����ˮCuSO4���������C���а�ɫ����������
��4����Ba��OH��2�ܽ�ȴ���Ca��OH��2���������CO2��BaCO3��Ħ����������CaCO3���������С����������Ϊǿ�CaCO3���ܽ�Ⱥ�BaCO3�ܽ������
�ʴ�Ϊ��AC��
��B�����յ���ˮ��ˮ�����ʵ���=$\frac{5.4g}{18g/mol}$=0.3mol��C�����յ��Ƕ�����̼����̼�ᱵ��ɫ����������̼ԭ���غ�ö�����̼�����ʵ���=$\frac{39.4g}{197g/mol}$=0.2mol������ͭ�����ʵ���=$\frac{54.2g-5.4g-0.2mol��44g/mol}{80g/mol}$=0.5mol����ͭ���ӡ����������Ӻ�̼������ӵ����ʵ���֮��=0.5mol��0.6mol��0.2mol=5��6��2�������仯ѧʽΪ��2CuCO3•3Cu��OH��2��CuCO3•3Cu��OH��2���ȷֽ�����CuO��������̼��ˮ���䷴Ӧ�ķ���Ϊ��2CuCO3•3Cu��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$5CuO+3H2O��+2CO2����
�ʴ�Ϊ��2CuCO3•3Cu��OH��2��3Cu��OH��2•2CuCO3��Cu5��OH��6��CO3��2��2CuCO3•3Cu��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$5CuO+3H2O��+2CO2����
���� ���⿼����̽�����ʵ���ɺͺ����IJⶨ����ȷ���ʵ�����̽��ʵ��Ļ������ѵ��ǻ�ѧʽ��ȷ�����Ѷ��еȣ������ڿ���ѧ���ķ������������������ͶԻ���֪ʶ���ۺ�Ӧ��������

 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д�| A�� | ������ȷ����ԭ�ӷ�������ṹ��������״������Ҫ���� | |
| B�� | ͨ����Ӧ�������������м������ݿ��Դ���Ԥ�ⷴӦ�ȵĴ�С | |
| C�� | ���ۼ��ļ���Խ����������ԽС�����ۻ�����Ҳ��Խ�ȶ� | |
| D�� | ͬ��ԭ�Ӽ��γɵĹ��ۼ�������������˫�������� |
| A�� | ���ܽ�����ݣ����ж����Mg��HCO3��2��Һ���ò�����Mg��OH��2����MgCO3 | |
| B�� | �÷е����ݣ����Ʋ��ܷ�һЩҺ�������÷���ķ������뿪���Ŀ����� | |
| C�� | �÷�Ӧ�����ݵĴ�С�����жϲ�ͬ��Ӧ�ķ�Ӧ���ʵĿ��� | |
| D�� | ��ԭ�Ӱ뾶���ݣ����ƶ�ijЩԭ�������Ի�ԭ�Ե�ǿ�� |
| A�� | �����ʵ���Ũ�ȵ�Na2CO3��NaHCO3���Һ�У�c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+�� | |
| B�� | 25��ʱ 0.2 mol•L-1����������0.05 mol��L-1Ba��OH��2��Һ��Ϻ���Һ��pH=l | |
| C�� | 25��ʱ��pH=3�Ķ�Ԫ����H2R��Һ��pH=ll��NaOH��Һ��Ϻ��Һ��pH����7����Ӧ��Ļ��Һ�У�2c��R2-��+c��HR-��=c��Na+�� | |
| D�� | 25��ʱ����0.3 mol��L-1 HY��Һ��0.3 mol��L-lNaOH��Һ�������Ϻ���Һ��pH=9����c��OH-��-c��HY��=1��lO-9 mol��L-1 |
| A�� | GFP��������ˮ������ˮ��Һ����ͨ����ֽ | |
| B�� | Ϊ�˷�ֹGFP���ʣ����������ڸ���������Һ�� | |
| C�� | GFP��һ�������»ᷢ��ˮ�⣬��ˮ�����һ��ֻ�Ц�-������ | |
| D�� | ����GFP������ս���ë����ζ |
| A�� | ���ֽⷴӦ | B�� | �û���Ӧ | C�� | ȡ����Ӧ | D�� | �ӳɷ�Ӧ |
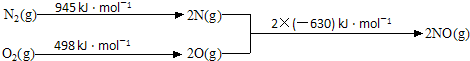


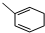
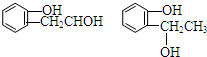 �ȣ�����FeCl3��Һ����ɫ�� ����Ũ������������ܷ�����ȥ��Ӧ��
�ȣ�����FeCl3��Һ����ɫ�� ����Ũ������������ܷ�����ȥ��Ӧ��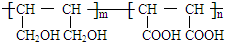 ��
�� ����CH2=CHCOOH�������Ʒ�Ӧ�ٵķ�Ӧ�����ɻ��������д������һ�ֽṹ��ʽΪ
����CH2=CHCOOH�������Ʒ�Ӧ�ٵķ�Ӧ�����ɻ��������д������һ�ֽṹ��ʽΪ ��
��