��Ŀ����
7��8.12���ը�¹��ֳ��е�NaCN�����ˮ�壬��ɻ�����Ⱦ�����������ɴ�����CN-�ķ�ˮ���ڼ��������£�Һ�Ƚ��軯�������������Σ��䶾�Խ�Ϊ�軯���ǧ��֮һ���������οɽ�һ����Һ������Ϊ�����ʣ��漰��Ӧ���£��������е�N��Ϊ-3�ۣ�����NaCN+2NaOH+Cl2=NaOCN+2NaCl+H2O
��NaOCN+NaOH+Cl2=CO2+N2+NaCl+H2O
��1������ƽ��Ӧ�ڵĻ�ѧ����ʽ2NaOCN+4NaOH+3Cl2=2CO2+N2��+6NaCl+2H2O
��2��ij��ˮ�к�NaCN����Ũ��Ϊ490mg/L���������������������������÷�ˮ20L��ʹNaCN��ȫת��Ϊ�����ʣ�������Һ��35.5 g��
���� NaOCN+NaOH+Cl2��CO2+N2+NaCl+H2O�У�NԪ�ػ��ϼ���-3������Ϊ0�ۣ�ClԪ�ػ��ϼ���0�۽���Ϊ-1�ۣ���ϵ����غ㼰ԭ���غ���ƽ�������ˮ��NaCN���������ٸ���n=$\frac{m}{M}$����NaCN�����ʵ������ɵ���ת���غ����n��Cl2�����ٸ���m=nM������Ҫ������������
��� �⣺NaOCN+NaOH+Cl2��CO2+N2+NaCl+H2O�У�NԪ�ػ��ϼ���-3������Ϊ0�ۣ�ClԪ�ػ��ϼ���0�۽���Ϊ-1�ۣ��ɵ����غ㼰ԭ���غ��֪���÷�ӦΪ2NaOCN+4NaOH+3Cl2=2CO2+N2��+6NaCl+2H2O����ˮ��NaCN������0.49g/L��20L=9.8g�����ʵ���Ϊn=$\frac{m}{M}$=$\frac{9.8g}{49g•mo{l}^{-1}}$=0.2mol����NaCN+2NaOH+Cl2=NaOCN+2NaCl+H2O��2NaOCN+4NaOH+3Cl2=2CO2+N2��+6NaCl+2H2O��֪��ʹNaCN��ȫת��Ϊ�����ʣ�Ӧ����CO��N2������������CԪ�ػ��ϼ���+2������Ϊ+4�ۣ�NԪ�ػ��ϼ���-3������Ϊ0�ۣ�ClԪ�ػ��ϼ���0�۽���Ϊ-1�ۣ���ѭ����ת���غ㣬��2��n��Cl2��=0.2mol����4-2��+0.2mol��[0-��-3��]�����n��Cl2��=0.5mol������Ҫ����������Ϊ0.5mol��71g/mol=35.5g��
�ʴ�Ϊ��2NaOCN+4NaOH+3Cl2=2CO2+N2��+6NaCl+2H2O��35.5��
���� ���⿼���˻�����Ⱦ��������������ԭ��Ӧ�����㣬���ظ�Ƶ����Ŀ��飬ע����Ŀ�е����غ��Ӧ�ã��ۺ��Խ�ǿ����Ŀ�Ѷ��еȣ�

| A�� | 75% | B�� | 13.6% | C�� | 14% | D�� | 25% |
| A�� | 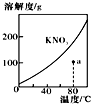 ��ʾKNO3���ܽ�����ߣ�a����ʾ����Һ��80��ʱKNO3��������Һ | |
| B�� |  ��ʾij���ȷ�Ӧ�ֱ����С�����������·�Ӧ�����е������仯 | |
| C�� |  ��ʾ0.1000mol/LNaOH�ζ�20.00 mL 0.1000mol/L����ĵζ����� | |
| D�� | 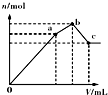 ��ʾ��NH4Al��SO4��2��Һ����ε���Ba��OH��2��Һ�����V�����n�ı仯 |
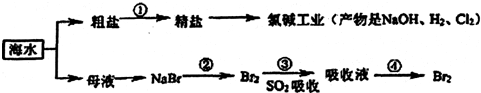
| A�� | ��ˮ��������Ҫ�����������������������ӽ������� | |
| B�� | �ڢٲ��г�ȥ�����п��������ʣ������μ���Na2CO3��Ba��OH��2��������Լ� | |
| C�� | �ڵڢڢۢܲ�����Ԫ�ؾ������� | |
| D�� | �ڢܲ��漰�ķ�������й��ˡ���ȡ������ |
| A�� | ��ϩ | B�� | ��Ȳ | C�� | ����ϩ | D�� | 1��3-����ϩ |
| A�� | ��10%��NaOH��Һ�У�ƽ��ÿ9��ˮ��������1��OH�� | |
| B�� | ��10g̼��Ʒ�ĩ��ˮ���Ƴ�100mL��Һ��CaCO3�����ʵ���Ũ��Ϊ1mol/L | |
| C�� | ͨ���״����11.2LHCl���壬��ʹ1L 0.5mol/L����������ʵ���Ũ������һ�� | |
| D�� | ��֪ij����������Һ��Na+��H2O�ĸ���֮��Ϊ1��a������������Һ���������Ƶ���������$\frac{20}{20+9a}$ |

| A�� | ʵ�����ȡ�İ����ܽ��ڱ���NaCl��Һ | |
| B�� | ʵ���ͨ���۲��ұ��Թ�����������˵�������Ƿ���������ʴ | |
| C�� | ʵ��ۿ�����֤��̼�ķǽ����Աȹ�ǿ | |
| D�� | ͼ����ʵ����ϴ�ӽ�ͷ�ιܵIJ��� |
| A�� | ���Ľ�����Ա���ǿ��������Ʒ������Ʒ������ʴ | |
| B�� | ˮ������������Ԫ����ͬ������ߵĻ�ѧ������ͬ | |
| C�� | Na+��Mg2+��Cl-��������������Ϊ8���ɴ˵ó����ӵ�������������Ϊ8 | |
| D�� | ͬ���·ֽ�����أ��Ӵ����ķ�Ӧ���ʸ��죬˵���������Ըı䷴Ӧ���� |
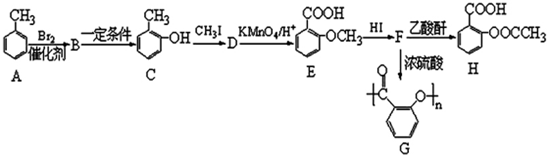
 ��B��C�ķ�Ӧ������ȡ����Ӧ��
��B��C�ķ�Ӧ������ȡ����Ӧ�� $��_{��}^{Ũ����}$
$��_{��}^{Ũ����}$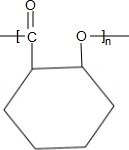 +nH2O��
+nH2O��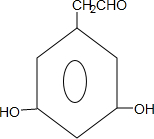 ��
�� ��
��