��Ŀ����
19���������������������ݣ��ó��Ľ�����ȷ���ǣ�������| A�� | ��10%��NaOH��Һ�У�ƽ��ÿ9��ˮ��������1��OH�� | |
| B�� | ��10g̼��Ʒ�ĩ��ˮ���Ƴ�100mL��Һ��CaCO3�����ʵ���Ũ��Ϊ1mol/L | |
| C�� | ͨ���״����11.2LHCl���壬��ʹ1L 0.5mol/L����������ʵ���Ũ������һ�� | |
| D�� | ��֪ij����������Һ��Na+��H2O�ĸ���֮��Ϊ1��a������������Һ���������Ƶ���������$\frac{20}{20+9a}$ |
���� A������ҺΪ100g������NaOH��H2O���������ٸ���n=$\frac{m}{M}$����������ʵ������н��
B��̼���������ˮ��
C��ͨ�백������Һ���������1L��
D��������������Һ����1molNa+����H2OΪamol��n��NaOH��=n��Na+��������m=nM����NaOH��H2O���������ٸ�����������=$\frac{m�����ʣ�}{m����Һ��}$��100%���㣮
��� �⣺A������ҺΪ100g��m��NaOH��=100g��10%=10g��m��H2O��=100g-10g=90g����n��OH-��=n��NaOH��=$\frac{10g}{40g/mol}$=0.25mol��n��H2O��=$\frac{90g}{18g/mol}$=5mol��������ˮ�ĵ��룬����Һ��ˮ������OH-�����ʵ���֮��Ϊ5mol��0.25mol=20��1����ƽ��ÿ20��ˮ��������1��OH-����A����
B��̼���������ˮ���ܽ��С��0.01g��10g̼��Ʋ�����ȫ�ܽ⣬���100mL��Һ��Ũ��ԭС��1mol/L����B����
C.1 LŨ��Ϊ0.5mol/L������ͨ������11.2L�������������ʵ���Ϊ1mol������Һ���������1L����C����
D���������������Һ����1molNa+����H2OΪamol��n��NaOH��=n��Na+��=1mol��m��NaOH��=1mol��40g/mol=40g��m��H2O��=amol��18g/mol=18a g����NaOH����������=$\frac{40g}{40g+18a}$=$\frac{20}{20+9a}$����D��ȷ��
��ѡD��
���� ���⿼�����ʵ���Ũ���������������йؼ��㣬��Ŀ�Ѷ��еȣ���ȷ���ʵ���Ũ�ȵĸ������ʽΪ���ؼ���Cѡ��Ϊ�״��㣬ѧ������ֻ����HCl�����ʵ�������������Һ�����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� Ŀ�����ϵ�д�
Ŀ�����ϵ�д�| A�� | Cl- | B�� | F - | C�� | S2- | D�� | Br |
| A�� | Na2O��Na2O2�����ڼ��������� | B�� | KOH��Na2CO3�����ڼ� | ||
| C�� | NaHSO4��HNO3�������� | D�� | Na2O��SiO2������������ |
| A�� | Ϊ��ȥ���еı��ӣ��ɼ���������ˮ��Һ���ٹ��� | |
| B�� | Ϊ��ȥFeSO4��Һ�е�Fe2��SO4��3���ɼ������ۣ��ٹ��� | |
| C�� | Ϊ��ȥ��Ȳ����������H2S����ʹ��ͨ��CuSO4��Һ | |
| D�� | Ϊ��ȥCO2��������SO2����ʹ��ͨ������NaHCO3��Һ |
| A�� | ���ԣ�H2SO4��H3PO4 | B�� | �ǽ����ԣ�O��S | ||
| C�� | ���ԣ�NaOH��Mg��OH��2 | D�� | �е㣺HF��HCl |
| A�� | c��S2-��+c��HS-��+c��H2S��+c��H+��=c��OH-�� | B�� | c��OH-��=c��HS-��+c��H+��+2 c��H2S�� | ||
| C�� | c��Na+��=c��S2-��+c��HS-��+c��H2S�� | D�� | c��Na+��+c��H+��=c��S2-��+c��HS-��+c��OH-�� |
��SO2 ��Fe3+ ��Fe2+ ��HCl ��H2O2��
| A�� | �٢ڢ� | B�� | �٢� | C�� | �٢ۢ� | D�� | �٢ۢܢ� |
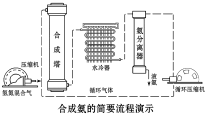 ���ڹ�����ռ�к���Ҫ�ĵ�λ������Լ��80%�İ��������컯�ʣ������������������������Ʒ��ԭ�ϣ����������������ǹ�ҵ�����������Ҫ������
���ڹ�����ռ�к���Ҫ�ĵ�λ������Լ��80%�İ��������컯�ʣ������������������������Ʒ��ԭ�ϣ����������������ǹ�ҵ�����������Ҫ������