ЬтФПФкШн
ЁОЬтФПЁПЙЄвЕЩЯГЃгУЬњжЪШнЦїЪЂзАРфХЈСђЫсЁЃЮЊбаОПЬњжЪВФСЯгыШШХЈСђЫсЕФЗДгІЃЌФГбЇЯАаЁзщНјааСЫвдЯТЬНОПЛюЖЏЃК
[ЬНОПвЛ]
ЃЈ1ЃЉНЋвбШЅГ§БэУцбѕЛЏЮяЕФЬњЖЄ(ЬМЫиИж)ЗХШыРфХЈСђЫсжаЃЌ10ЗжжгКѓвЦШыСђЫсЭШмвКжаЃЌЦЌПЬКѓШЁГіЙлВьЃЌЬњЖЄБэУцЮоУїЯдБфЛЏЃЌЦфдвђЪЧ__________________ЁЃ
ЃЈ2ЃЉСэГЦШЁЬњЖЄ6.0 gЗХШы15.0 mLХЈСђЫсжаЃЌМгШШЃЌГфЗжЗДгІКѓЕУЕНШмвКXВЂЪеМЏЕНЦјЬхYЁЃ
ЂйМзЭЌбЇШЯЮЊXжаГ§Fe3ЃЋЭтЛЙПЩФмКЌгаFe2ЃЋЁЃШєвЊШЗШЯЦфжаЕФFe2ЃЋЃЌгІбЁгУ_________ЁЃ
aЃЎKSCNШмвККЭТШЫЎЁЁ bЃЎЬњЗлКЭKSCNШмвК
cЃЎХЈАБЫЎ dЃЎЫсадKMnO4ШмвК
ЂкввЭЌбЇШЁ448 mL(БъзМзДПі)ЦјЬхYЭЈШызуСПфхЫЎжаЃЌЗЂЩњЯТСаЗДгІЃКSO2ЃЋBr2ЃЋ2H2O===2HBrЃЋH2SO4ЃЌШЛКѓМгШызуСПBaCl2ШмвКЃЌОЪЪЕБВйзїКѓЕУИЩдяЙЬЬх2.33 gЁЃгЩДЫЭЦжЊЦјЬхYжаSO2ЕФЬхЛ§ЗжЪ§ЮЊ________ЁЃ
[ЬНОПЖў] ЗжЮіЩЯЪіЪЕбщжаSO2ЬхЛ§ЗжЪ§ЕФНсЙћЃЌБћЭЌбЇШЯЮЊЦјЬхYжаЛЙПЩФмКЌгаH2КЭQЦјЬхЁЃЮЊДЫЩшМЦСЫЯТСаЬНОПЪЕбщзАжУ(ЭМжаМаГжвЧЦїЪЁТд)ЁЃ
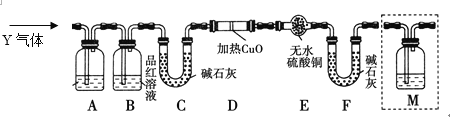
ЃЈ3ЃЉзАжУBЕФзїгУЪЧ__________________________________________________ЁЃ
ЃЈ4ЃЉШЯЮЊЦјЬхYжаЛЙКЌгаQЕФРэгЩЪЧ____________________ (гУЛЏбЇЗНГЬЪНБэЪО)ЁЃ
ЃЈ5ЃЉЮЊШЗШЯQЕФДцдкЃЌашвЊгУЕНзАжУMЃЌдђMжазАЕФЪдМСЪЧ______________ЃЌВЂНЋMЬэМггк________(бЁЬюађКХ)ЁЃ
aЃЎAжЎЧА bЃЎAЃBМф cЃЎBЃCМф dЃЎCЃDМф
ЃЈ6ЃЉШчЙћЦјЬхYжаКЌгаH2ЃЌдЄМЦЪЕбщЯжЯѓгІЪЧ_____________________________ЁЃ
ЃЈ7ЃЉШєФГЦјЬхПЩФмгЩCO2ЁЂSO2ЁЂH2жаЕФвЛжжЛђЖржжзщГЩЃЌОВтЖЈЦфжабѕЕФжЪСПЗжЪ§ЮЊ50%ЃЌдђИУЦјЬхЕФзщГЩПЩФмЮЊ___________ЃЈЬюађКХЃЉЁЃ
aЃЎSO2 bЃЎH2ЁЂSO2 cЃЎH2ЁЂCO2 dЃЎCO2ЁЂSO2 eЃЎSO2ЁЂCO2ЁЂH2
ЁОД№АИЁП ЬњЖЄБэУцБЛЖлЛЏ d 50% МьбщSO2ЪЧЗёГ§ОЁ CЃЋ2H2SO4(ХЈ)![]() CO2ЁќЃЋ2SO2ЁќЃЋ2H2O ГЮЧхЪЏЛвЫЎ c DжаЙЬЬхгЩКкБфКьКЭEжаЙЬЬхгЩАзБфРЖ ace
CO2ЁќЃЋ2SO2ЁќЃЋ2H2O ГЮЧхЪЏЛвЫЎ c DжаЙЬЬхгЩКкБфКьКЭEжаЙЬЬхгЩАзБфРЖ ace
ЁОНтЮіЁПЃЈ1ЃЉЬњЖЄЗХШыРфСђЫсжаЃЌХЈСђЫсгаНЯЧПЕФбѕЛЏадФмЪЙЬњЖЄЖлЛЏзшжЙЗДгІНјвЛВННјааЃЛе§ШЗД№АИЃКЬњЖЄБэУцБЛЖлЛЏЁЃ
ЃЈ2ЃЉЂйбЧЬњРызгФмЪЙЫсадИпУЬЫсМиЭЪЩЋЃЌШмвКжавбОгаШ§МлЬњРызгЃЌбЁдёaЛсдьГЩИЩШХЃЛbФмМьбщШ§МлЬњРызгЕФДцдкЃЛбЁcЩњГЩСНжжГСЕэЃЌЪмЧтбѕЛЏЬњГСЕэбеЩЋЕФгАЯьЮоЗЈЗжБцЃЛFe2+гыЫсадKMnO4ШмвКЗЂЩњбѕЛЏЛЙдЗДгІЃЌFe2+ФмЪЙЫсадKMnO4ШмвКЭЪЩЋЃЌе§ШЗбЁЯюdЁЃ
ЂкSO2ОпгаЛЙдадЃЌЭЈШызуСПфхЫЎжаЃЌЗЂЩњSO2+Br2+2H2O=2HBr+H2SO4ЃЌЩњГЩЕФСђЫсгіЕНТШЛЏБЕЛсВњЩњАзЩЋГСЕэЃЌдђnЃЈЛьКЯЦјЬхЃЉ=0.448LЁТ22.4L/mol=0.02molЃЌИљОнЖдгІЙиЯЕЃКSO2ЁЋBaSO4ЃЌ nЃЈSO2ЃЉ=nЃЈBaSO4ЃЉ=2.33gЁТ233g/mol=0.01 molЃЌНтЕУnЃЈSO2ЃЉ=0.01molЃЌЫљвдЖўбѕЛЏСђЕФЬхЛ§ЗжЪ§ЮЊЃК0.01molЁТ0.02molЁС100%=50%ЃЛе§ШЗД№АИЃК50%ЁЃ
ЃЈ3ЃЉAГ§ШЅЖўбѕЛЏСђЃЌЖўбѕЛЏСђФмЪЙЦЗКьШмвКЭЪЩЋЃЌЫљвдBПЩвдМьбщAжаЪЧЗёЭъШЋГ§ШЅЖўбѕЛЏСђЃЛе§ШЗД№АИЃКМьбщSO2ЪЧЗёГ§ОЁЁЃ
ЃЈ4ЃЉдкМгШШЪБЃЌЬњЖЄжаВЛНіЬњКЭХЈСђЫсЗДгІЃЌЬМвВКЭХЈСђЫсЗДгІЩњГЩЩњГЩЖўбѕЛЏСђЁЂЖўбѕЛЏЬМКЭЫЎЃЌЗДгІЗНГЬЪНЮЊЃКCЃЋ2H2SO4(ХЈ)![]() CO2ЁќЃЋ2SO2ЁќЃЋ2H2OЃЛе§ШЗД№АИЃКCЃЋ2H2SO4(ХЈ)
CO2ЁќЃЋ2SO2ЁќЃЋ2H2OЃЛе§ШЗД№АИЃКCЃЋ2H2SO4(ХЈ)![]() CO2ЁќЃЋ2SO2ЁќЃЋ2H2OЁЃ
CO2ЁќЃЋ2SO2ЁќЃЋ2H2OЁЃ
ЃЈ5ЃЉQЮЊЖўбѕЛЏЬМЃЌЖўбѕЛЏЬМКЭЖўбѕЛЏСђЖМФмЪЙГЮЧхЪЏЛвЫЎБфЛызЧЃЌбЁдёaЛђbЪмЖўбѕЛЏСђЕФгАЯьЮоЗЈХаЖЯЖўбѕЛЏЬМЕФДцдкЃЌбЁdЪБЖўбѕЛЏЬМБЛМюЪЏЛвЮќЪеЃЌBЃCМфМгШыГЮЧхЪЏЛвЫЎПЩМьбщCO2ЃЛе§ШЗД№АИЃКГЮЧхЪЏЛвЫЎЃЛcЁЃ
ЃЈ6ЃЉЧтЦјЛЙдбѕЛЏЭЛсЩњГЩЫЎеєЦјФмЪЙАзЩЋЕФСђЫсЭЗлФЉБфРЖЩЋЃЌЭЌЪБгаКьЩЋЕФЭЕЅжЪЩњГЩЃЌе§ШЗД№АИЃКDжаЙЬЬхгЩКкЩЋБфКьКЭEжаЙЬЬхгЩАзБфРЖЁЃ
ЃЈ7ЃЉCO2жаІиЃЈOЃЉ=32/44ЁС100%=72%, SO2жаІиЃЈOЃЉ=32/64ЁС100%=50%ЃЛШєФГЦјЬхПЩФмгЩCO2ЁЂSO2ЁЂH2жаЕФвЛжжЛђЖржжзщГЩЃЌОВтЖЈЦфжабѕЕФжЪСПЗжЪ§ЮЊ50%ЃЌдђИУЦјЬхЕФзщГЩжЛгаSO2ЃЌбѕЕФжЪСПЗжЪ§ЮЊ50%ЃЌaе§ШЗЃЛH2КЭSO2ЛьКЯЦјЬхжабѕЕФжЪСПЗжЪ§аЁгк50%ЃЌbДэЮѓЃЛH2КЭCO2ЛьКЯЦјЬхжабѕЕФжЪСПЗжЪ§ПЩвдЮЊ50%ЃЌcе§ШЗЃЛCO2КЭSO2КЯЦјЬхжабѕЕФжЪСПЗжЪ§Дѓгк50%ЃЌdДэЮѓЃЛSO2ЁЂCO2КЭH2ЛьКЯЦјЬхжабѕЕФжЪСПЗжЪ§ПЩвдЕШгк50%ЃЌeе§ШЗЃЛе§ШЗбЁЯюaceЁЃ

 ЛЅЖЏгЂгяЯЕСаД№АИ
ЛЅЖЏгЂгяЯЕСаД№АИ