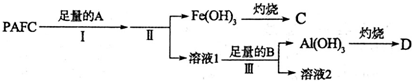
��Ŀ����
13����һ��ʯ��ʯ��Ʒ�����к��е������Ƕ������裨����һ�ֲ�����ˮ��Ҳ�������ᷴӦ�����µĹ������ʣ���ijͬѧ��ⶨ����Ʒ�Ĵ��ȣ���ȡ��2g����ʯ��ʯ��Ʒ����20gϡ������Ĵμ��룬��ַ�Ӧ��ʣ�������������±���ʾ��| ϡ��������� | ʣ���������� |
| ��1���5g | 1.315g |
| ��2���5g | 0.63g |
| ��3���5g | 0.3g |
| ��4���5g | 0.3g |
��2��ʯ��ʯ��Ʒ�Ĵ���Ϊ��
��3��100Kg���ִ��ȵ�ʯ��ʯ������պɵõ�������ٿˣ�
���� ��1��������Ʒ�г�̼����⣬����ijɷּȲ������ᷴӦ��Ҳ���ܽ���ˮ������ɱ���ÿ�μ���5gϡ���������ٵ�������ϵ���жϣ�ÿ����5gϡ�������Ӧ����0.685g���ݴ˹��ɣ��ɵ��Ĵμ����������������ļ���ֵС��0.685g���жϴ˴η�Ӧ��̼�������ȫ��Ӧ��
��2��������Ʒ���ʵ��������ɴ����ʯ��ʯ��Ʒ��̼��Ƶ�����������
��3������̼��Ƹ����·ֽⷴӦ�Ļ�ѧ����ʽ��ȷ���ֽ��̼�����ų�������̼��������ϵ������ʯ��ʯ��̼��Ƶ�������������ܵõ�������̼�����������
��� �⣺��1�����ݱ��е�ʵ�����ݿɵã�ÿ����5gϡ���ᣬ������������0.685g�������μ���ϡ���������ٵ�����=0.63g-0.3g=0.33g��С��0.685g������ʱ̼�������ȫ��Ӧ������ʣ���0.3g���弴Ϊ���ʵ���������2gʯ��ʯ��Ʒ�е�����������0.3g��
��2��ʯ��ʯ��Ʒ��̼��Ƶ���������=$\frac{2g-0.3g}{2g}$��100%=85%����ʯ��ʯ��Ʒ�Ĵ���Ϊ85%��
��3�����ܵõ�������̼������Ϊx
CaCO3 $\frac{\underline{\;����\;}}{\;}$ CaO+CO2��
100 44
100g��85% x
$\frac{100}{100g��85%}=\frac{44}{x}$
��ã�x=37.4g�����Կɵõ�����Ϊ100-37.4=62.6g��
�𣺿ɵõ�����62.6g��
���� ����ͼ����ʣ����������������ÿ�μ�����ͬϡ���������ٵ���������Ʒ�б���Ӧ��̼��Ƶ��������жϳ����Ĵμ�ϡ�����̼�����ȫ��Ӧ����Ϊ�����ͻ�ƿڣ�

| A�� | ���ֲ�ͬԪ�ص�ԭ�� | B�� | ���ֲ�ͬԪ�ص����� | ||
| C�� | ���ֲ�ͬ���� | D�� | ����Ԫ�ص�ԭ�Ӻ����� |
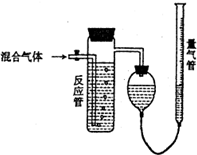 ����SO2��N2��O2���������SO2������װ����ͼ����Ӧ����װ�е�ĵ�����Һ��SO2��I2�����ķ�ӦΪ��N2��O2����I2��Ӧ����SO2+I2+2H2O��H2SO4+2HI����Ӧ������Һ��ɫ��ʧ��û�м�ʱֹͣͨ������õ�SO2�����������������
����SO2��N2��O2���������SO2������װ����ͼ����Ӧ����װ�е�ĵ�����Һ��SO2��I2�����ķ�ӦΪ��N2��O2����I2��Ӧ����SO2+I2+2H2O��H2SO4+2HI����Ӧ������Һ��ɫ��ʧ��û�м�ʱֹͣͨ������õ�SO2�����������������| A�� | ƫ�� | B�� | ƫ�� | C�� | ��Ӱ�� | D�� | ��ȷ�� |
| A�� | ������ˮ�ĵ����һ����ǿ����ʣ�������ˮ�ĵ����һ����������� | |
| B�� | ǿ�������Һ�ĵ�������һ�������������Һǿ | |
| C�� | NaCl��Һ�ڵ����������µ���������Ӻ������� | |
| D�� | �Ȼ��ƾ��岻�����������Ȼ��ƾ����в����������ƶ������� |
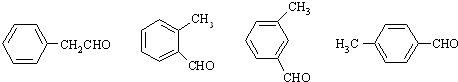 ����ѡһ�֣���
����ѡһ�֣���
 ����������γɵĻ�����ĽṹʽΪ��
����������γɵĻ�����ĽṹʽΪ�� ��
�� ��
��