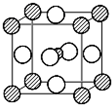
��Ŀ����
2�����������������������й㷺Ӧ�ã������պʹ���Ҳʮ����Ҫ����1��SO2���л�ԭ�ԣ�д����SO2����ͨ�� FeCl3��Һ�е����ӷ���ʽ�����������ת�Ƶ���Ŀ������
2Fe3++SO2+2H2O=2Fe2++4H++SO42-��

��2����Na2SO3��Һ�еμӷ�̪����Һ���ɫ�����ڸ���Һ�е��������BaCl2��Һ�������Dz�����ɫ�������Һ�ɫ��ȥ���������ӷ���ʽ������ƽ��ԭ�����н�����Na2SO3��Һ�У�SO32-ˮ��SO32-+H2O?HSO3-+OH-������BaCl2��Ba2++SO32-=BaSO3������ɫ��������c��SO32-����С��SO32-ˮ��ƽ�����ƣ�c��OH-����С����̪��ɫ��
��3������������ʵ���Ũ�ȵ� NaClO��Һ��Na2SO3��Һ��Ϻ���Һ�����ԣ���ʱ��Һ��Ũ����ȵ�����H+��OH-��SO42-��Cl-��
��4����֪��H2S��Ki1=1.3��10-7 Ki2=7.1��10-15H2CO3��Ki1=4.3��10-7 Ki2=5.6��10-11����H2Sβ����������Na2CO3��Һ�����գ�д�����ӷ�Ӧ����ʽ��H2S+CO32-=HS-+HCO3-������ʱ���������Ũ�ȵ�Na2S��Na2CO3��Һ������������Nǰ��N�����������������
���� ��1��SO2��FeCl3����Ϊ��������ӣ�FeCl3����ԭΪ�������ӣ��ݻ��ϼ۱仯�����ת�ƣ�
��2��Na2SO3��Һ��BaCl2��Һ��Ӧ����BaSO3����ʹSO32-ˮ��ƽ�����ƣ�c��OH-����С����̪��ɫ��
��3��ClO-�������ԣ��ܹ�����SO32-����Ӧ�����ӷ���ʽΪClO-+SO32-=Cl-+SO42-������������ʵ���Ũ�ȵ� NaClO��Һ��Na2SO3��Һ��Ϻ�������ͬŨ�ȵ�NaCl��Na2SO4�����Һ��
��4��������ĵ���ƽ�ⳣ����֪������H2CO3��H2S��HCO3-��HS-������Խ�����������ˮ��̶�Խǿ����ϵ���غ������
��� �⣺��1��SO2���л�ԭ�ԣ���SO2����ͨ�� FeCl3��Һ�У�SO2��FeCl3����Ϊ��������ӣ�FeCl3����ԭΪ�������ӣ���Ӧ�����ӷ���ʽΪ2Fe3++SO2+2H2O=2Fe2++4H++SO42-��ÿ��1molSO2��Ӧת��2mol���ӣ�����ת�Ƶ���Ŀ������Ϊ ���ʴ�Ϊ��2Fe3++SO2+2H2O=2Fe2++4H++SO42-��
���ʴ�Ϊ��2Fe3++SO2+2H2O=2Fe2++4H++SO42-�� ��
��
��2��Na2SO3��Һ��BaCl2��Һ��Ӧ����BaSO3������ʹSO32-ˮ��ƽ��SO32-+H2O?HSO3-+OH-���ƣ�c��OH-����С�����Է�̪��ɫ��
�ʴ�Ϊ��������ɫ�������Һ�ɫ��ȥ����Na2SO3��Һ�У�SO32-ˮ��SO32-+H2O?HSO3-+OH-������BaCl2��Ba2++SO32-=BaSO3������ɫ��������c��SO32-����С��SO32-ˮ��ƽ�����ƣ�c��OH-����С����̪��ɫ��
��3��ClO-�������ԣ��ܹ�����SO32-����Ӧ�����ӷ���ʽΪClO-+SO32-=Cl-+SO42-������������ʵ���Ũ�ȵ� NaClO��Һ��Na2SO3��Һ��Ϻ�������ͬŨ�ȵ�NaCl��Na2SO4�����Һ����Һ�����ԣ�H+��OH-��SO42-��Cl-����Ũ����ȣ��ʴ�Ϊ���У�H+��OH-��SO42-��Cl-��
��4��������ĵ���ƽ�ⳣ����֪������H2CO3��H2S��HCO3-��HS-��H2S��������Na2CO3��Һ��Ӧ�����ӷ���ʽΪ��H2S+CO32-=HS-+HCO3-��HCO3-�����Ա�HS-ǿ��Խ��Խˮ�⣬����Na2S��Һ��Na2CO3��Һ�ļ���ǿ��c��H+��С���ݵ���غ���̼������Һ����c��Na+��+c��H+��=c��OH-��+c��HCO3-��+2c��CO32-������������Һ����c��Na+��+c��H+��=c��OH-��+c��HS-��+2c��S2-������Ũ�ȵ�����Һ�У�c��Na+����ͬ��S2-��ˮ��������CO32-��ˮ��̶ȴ����ˮ����������Ӹ��࣬��˵����ʱ��Na2S��Na2CO3��Һ������������Nǰ��N�����ʴ�Ϊ��H2S+CO32-=HS-+HCO3-������
���� ���⿼����������ԭ��Ӧ����ʽ��д������ˮ��ƽ���ƶ�����Һ������жϡ�����Ũ�ȴ�С�Ƚϣ���Ŀ�ѶȽϴ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� |
 ��
����2��������������ˮ�����У�������ǿ�Ļ�����ķ���ʽ��HClO4��������ǿ�Ļ�������ǣ�KOH��
��3������������������������Ԫ����Al��д����������������Ӧ��ˮ�������������Ʒ�Ӧ�����ӷ���ʽAl��OH��3+OH-=AlO2-+2H2O��
��4���õ���ʽ��ʾԪ�آ���Ļ�������γɹ��̣�
 ���û������������ӣ�����ۡ������ӡ��������
���û������������ӣ�����ۡ������ӡ����������5��Ԫ�ص���̬�⻯���гɼ��Ե�������NH3����Ԫ���⻯����������������Ӧˮ���ﷴӦ�Ļ�ѧ����ʽΪNH3+HNO3=NH4NO3��
| ϡ��������� | ʣ���������� |
| ��1���5g | 1.315g |
| ��2���5g | 0.63g |
| ��3���5g | 0.3g |
| ��4���5g | 0.3g |
��2��ʯ��ʯ��Ʒ�Ĵ���Ϊ��
��3��100Kg���ִ��ȵ�ʯ��ʯ������պɵõ�������ٿˣ�
| A�� | Ϊ��ֹҩƷ���������ϣ���ͷ�ιܿ������Թ��ڲ��μ�Һ�� | |
| B�� | ����Ͳȡ13.37mL��ϡ���� | |
| C�� | ���Թ���Һ�����ʱ��Һ������������Թ��ݻ���1/4 | |
| D�� | ��ƾ��������Ӿƾ�ʱ�����ܳ����ƾ����ݻ���2/3 |
| A�� | ��0.1 mol/L��pH=1��NaHA��Һ�м���NaOH��Һ��H++OH-=H2O | |
| B�� | ϡ�����м���������ۣ�Fe+4H++NO3-�TFe3++NO��+2H2O | |
| C�� | ��ʾ����������������Ӧ���к��ȵ��Ȼ�ѧ��Ӧ����ʽΪ��$\frac{1}{2}$ H2SO4��aq��+$\frac{1}{2}$ Ba��OH��2��aq��=$\frac{1}{2}$ BaSO4��s��+H2O��l����H=-57.3 kJ/mol | |
| D�� | NH4Al��SO4��2��Һ�м���Ba��OH��2��ҺʹSO42-��ȫ������Al3++2SO42-+2Ba2++4OH-=AlO2-+2BaSO4��+2H2O |
| A�� | 5.6g��������������ȫ��Ӧʱʧȥ�ĵ�����ĿΪ0.2NA | |
| B�� | ��״���£�11.2L H2�к��еĵ�����Ϊ1NA | |
| C�� | ��״���£�2.24 Lˮ�к���ˮ����0.1 NA | |
| D�� | 2L1mol/L�������������Ȼ��������Ϊ2NA |
| A�� | 14C��������������������14C��13C��Ϊͬ�������� | |
| B�� | ����������Ϊ2��Ԫ��һ���ǽ���Ԫ�� | |
| C�� | ����Ԫ��һ���ǽ���Ԫ�� | |
| D�� | ͬһ�����еĵڢ�A��͵ڢ�A��Ԫ�ص�ԭ��������һ��Ϊ1��11 |