��Ŀ����
����Ŀ����T��ʱ��AgBr��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����֪T��ʱAgCl��Ksp=2��10 -10������˵������ȷ����( )
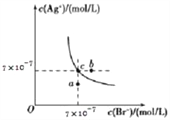
A. ��T��ʱ��AgBr��KspΪ4.9��10-13
B. ��AgBr�ı�����Һ�м���NaBr���壬��ʹ��Һ��c�㵽b��
C. ͼ��a���Ӧ���Ǻ�AgBr�IJ�������Һ
D. ��T��ʱ��AgCl��s��+Br -��aq��![]() AgBr��s��+Cl -��aq����ƽ�ⳣ��K��408
AgBr��s��+Cl -��aq����ƽ�ⳣ��K��408
���𰸡�B
��������A. ��ͼ��֪����T��ʱ��AgBr��Ksp= c(Ag��)��c(Br��)=7��10��7��7��10��7=4.9��10��13����A��ȷ��B. ��AgBr�ı�����Һ�д����ܽ�ƽ�⣺AgBr(s) ![]() Ag��(aq)+Br��(aq)������NaBr���壬c(Br��)����AgBr���ܽ�ƽ�������ƶ���c(Ag��)��С�����Բ�����ʹ��Һ��c��䵽b�㣬��B����C. ͼ��a���Ӧ��Qc(AgBr)��Ksp(AgBr)������a���Ӧ����AgBr�IJ�������Һ����C��ȷ��D. ��T��ʱ��AgCl��s��+Br-��aq��
Ag��(aq)+Br��(aq)������NaBr���壬c(Br��)����AgBr���ܽ�ƽ�������ƶ���c(Ag��)��С�����Բ�����ʹ��Һ��c��䵽b�㣬��B����C. ͼ��a���Ӧ��Qc(AgBr)��Ksp(AgBr)������a���Ӧ����AgBr�IJ�������Һ����C��ȷ��D. ��T��ʱ��AgCl��s��+Br-��aq��![]() AgBr��s��+Cl-��aq����ƽ�ⳣ��K=
AgBr��s��+Cl-��aq����ƽ�ⳣ��K= ![]() =
= ![]() =
= ![]() =
= ![]() ��408����D��ȷ����ѡB��
��408����D��ȷ����ѡB��

����Ŀ��10��ʱ����NaHCO3������Һ����ø���Һ��pH�������±仯��
�¶ȣ��棩 | 10 | 20 | 30 | ������к���ȴ��50�� |
pH | 8.3 | 8.4 | 8.5 | 8.8 |
�����������ݣ���ͬѧ��Ϊ������Һ��pH���ߵ�ԭ����HCO3����ˮ��̶����ʼ�����ǿ���÷�Ӧ�����ӷ���ʽΪ____________________________����ͬѧ��Ϊ����ҺpH���ߵ�ԭ����NaHCO3���ȷֽ⣬������Na2CO3�����ƶ�Na2CO3��ˮ��̶�_______����������������С������NaHCO3����ͬѧ��Ϊ�ס��ҵ��ж϶�����֡�����Ϊ��
��1��ֻҪ�ڼ�����е���Һ�м����������Լ�X����������������____�������������������ж���ȷ���Լ�X��_____________������ţ���
A��Ba(OH)2��Һ B��BaCl2��Һ C��NaOH��Һ D�������ʯ��ˮ
��2�������Ⱥ����Һ��ȴ��10��������Һ��pH_____(��������������������������������8.3����_____���������������������ж���ȷ��
��3���������ϣ�����NaHCO3�ķֽ��¶�Ϊ150����������_____���������������������ж��Ǵ���ģ�������_____________________________________________________��
����Ŀ�����ݱ�����Ϣ�жϣ�����ѡ����ȷ���� (�� ��)
��� | ��Ӧ�� | ���� |
�� | KMnO4��H2O2��H2SO4 | K2SO4��MnSO4�� |
�� | Cl2��FeBr2 | FeCl3��FeBr3 |
�� | MnO | Cl2��Mn2���� |
A. �ڢ��鷴Ӧ���������ֻ��O2
B. �ڢ��鷴Ӧ��Cl2��FeBr2�����ʵ���֮��Ϊ1��2
C. �ڢ��鷴Ӧ������1 mol Cl2��ת�Ƶ���10 mol
D. ��������ǿ����˳��ΪMnO![]() ��Cl2��Fe3����Br2
��Cl2��Fe3����Br2