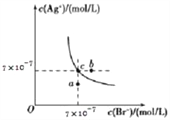
МвДҝДЪИЭ
ЎҫМвДҝЎҝЎӘ¶ЁОВ¶ИПВЈ¬Ҫ«2molSO2әНlmolO2ідИл10LәгИЭГЬұХИЭЖчЦРЈ¬·ўЙъ·ҙУҰ 2SO2(g) +O2(g) ![]() 2SO3(g) ЎчH=-196 kJmol-1Ј¬5minКұҙпөҪЖҪәвЈ¬ІвөГ·ҙУҰ·ЕИИ166.6 kJЎЈПВБРЛө·ЁҙнОуөДКЗ
2SO3(g) ЎчH=-196 kJmol-1Ј¬5minКұҙпөҪЖҪәвЈ¬ІвөГ·ҙУҰ·ЕИИ166.6 kJЎЈПВБРЛө·ЁҙнОуөДКЗ
A. 05 minДЪЈ¬УГO2ұнКҫөДЖҪҫщ·ҙУҰЛЩВКv(O2)=0.017 molL-1 min-1
B. ![]() өДЦөІ»ұдКұЈ¬ёГ·ҙУҰҙпөҪЖҪәвЧҙМ¬
өДЦөІ»ұдКұЈ¬ёГ·ҙУҰҙпөҪЖҪәвЧҙМ¬
C. ИфФцҙуO2өДЕЁ¶ИЈ¬SO2өДЧӘ»ҜВКФцҙу
D. МхјюІ»ұдЈ¬ЖрКјПтИЭЖчЦРідИл4 mol SO2әН 2 mol O2Ј¬ЖҪәвКұ·ЕИИРЎУЪ333.2 kJ
Ўҫҙр°ёЎҝD
ЎҫҪвОцЎҝ
AЈ®ОпЦКөДБҝУлИИБҝіЙХэұИЈ¬5minКұҙпөҪЖҪәвЈ¬ІвөГ·ҙУҰ·ЕИИ166.6kJЈ¬ФтІОјУ·ҙУҰөДСхЖшОӘ![]() =0.85molЈ¬0Ў«5minДЪЈ¬УГO2ұнКҫөДЖҪҫщ·ҙУҰЛЩВКv(O2)=
=0.85molЈ¬0Ў«5minДЪЈ¬УГO2ұнКҫөДЖҪҫщ·ҙУҰЛЩВКv(O2)=![]() =0.017molL-1min-1Ј¬№КAХэИ·Ј»B.
=0.017molL-1min-1Ј¬№КAХэИ·Ј»B.![]() өДұИЦөІ»ұдКұЈ¬ҝЙЦӘёчОпЦКөДОпЦКөДБҝІ»ұдЈ¬Фт·ҙУҰҙпөҪЖҪәвЈ¬№КBХэИ·Ј»CЈ®ИфФцҙуO2өДЕЁ¶ИЈ¬ЖҪәвХэПтТЖ¶ҜЈ¬ҙЩҪш¶юСх»ҜБтөДЧӘ»ҜЈ¬ФтSO2өДЧӘ»ҜВКФцҙ󣬹КCХэИ·Ј»D.2molSO2әН1molO2ідИл10LәгИЭГЬұХИЭЖчЦРҙпөҪЖҪәвКұЈ¬ПыәДСхЖшОӘ0.85molЈ¬·ЕіцИИБҝОӘ166.6kJЈ¬¶шМхјюІ»ұдЈ¬ЖрКјПтИЭЖчЦРідИл4molSO2әН2molO2Ј¬ОпЦКөДБҝОӘФӯАҙөД2ұ¶Ј¬С№ЗҝФцҙуЈ¬ЖҪәвХэПтТЖ¶ҜЈ¬ҙпөҪЖҪәвКұПыәДСхЖшҙуУЪ0.85molЎБ2=1.7molЈ¬ФтЖҪәвКұ·ЕИИҙуУЪ166.6kJЎБ2=333.2kJЈ¬№КDҙнОуЈ»№КСЎDЎЈ
өДұИЦөІ»ұдКұЈ¬ҝЙЦӘёчОпЦКөДОпЦКөДБҝІ»ұдЈ¬Фт·ҙУҰҙпөҪЖҪәвЈ¬№КBХэИ·Ј»CЈ®ИфФцҙуO2өДЕЁ¶ИЈ¬ЖҪәвХэПтТЖ¶ҜЈ¬ҙЩҪш¶юСх»ҜБтөДЧӘ»ҜЈ¬ФтSO2өДЧӘ»ҜВКФцҙ󣬹КCХэИ·Ј»D.2molSO2әН1molO2ідИл10LәгИЭГЬұХИЭЖчЦРҙпөҪЖҪәвКұЈ¬ПыәДСхЖшОӘ0.85molЈ¬·ЕіцИИБҝОӘ166.6kJЈ¬¶шМхјюІ»ұдЈ¬ЖрКјПтИЭЖчЦРідИл4molSO2әН2molO2Ј¬ОпЦКөДБҝОӘФӯАҙөД2ұ¶Ј¬С№ЗҝФцҙуЈ¬ЖҪәвХэПтТЖ¶ҜЈ¬ҙпөҪЖҪәвКұПыәДСхЖшҙуУЪ0.85molЎБ2=1.7molЈ¬ФтЖҪәвКұ·ЕИИҙуУЪ166.6kJЎБ2=333.2kJЈ¬№КDҙнОуЈ»№КСЎDЎЈ

ЎҫМвДҝЎҝУГИзНјЧ°ЦГАҙ·ЦАлCO2әНCO»мәПЖшМеІўёЙФпЈ¬НјЦРaЎўcЎўdОӘЦ№Л®јРЈ¬bОӘ·ЦТәВ©¶·»оИыЈ¬НЁ№эYРО№ЬәНЦ№Л®јР·ЦұрҪУБҪёцЗтөЁЈ¬ПЦЧ°ЦГДЪҝХЖшТСЕЕҫЎЈ¬ОӘК№КөСйіЙ№ҰЈ¬јЧЎўТТЎўұы·ЦұрКў·ЕөДКФјБОӘЈЁЈ©
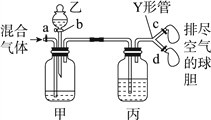
јЧ | ТТ | ұы | |
A | ұҘәНNaHCO3ИЬТә | 12 molЎӨLЈӯ1СОЛб | 18.4 molЎӨLЈӯ1 H2SO4 |
B | ұҘәНNa2CO3ИЬТә | 2 molЎӨLЈӯ1 H2SO4 | ұҘәНNaOHИЬТә |
C | ұҘәНNaOHИЬТә | 2 molЎӨLЈӯ1 H2SO4 | 18.4 molЎӨLЈӯ1 H2SO4 |
D | 18.4 molЎӨLЈӯ1 H2SO4 | ұҘәНNaOHИЬТә | 18.4 molЎӨLЈӯ1 H2SO4 |
A. A B. B C. C D. D
ЎҫМвДҝЎҝМјәНМјөД»ҜәПОпФЪЙъІъЎўЙъ»оЦРөДУҰУГ·ЗіЈ№г·әЎЈ ПЦҪ«І»Н¬БҝөДCO2ЈЁgЈ©әНH2ЈЁgЈ©·ЦұрНЁИлМе»эОӘ2LөДәгИЭГЬұХИЭЖчЦРЈ¬ҪшРРИзПВ·ҙУҰЈәCO2(gЈ©+H2ЈЁgЈ©![]() [Failed to download image : ]COЈЁgЈ©+H2OЈЁgЈ©Ј¬өГөҪИзПВИэЧйКэҫЭЈә
[Failed to download image : ]COЈЁgЈ©+H2OЈЁgЈ©Ј¬өГөҪИзПВИэЧйКэҫЭЈә
КөСйЧй | ОВ¶И/Ўж | ЖрКјБҝ/mol | ЖҪәвБҝ/mol | ҙпөҪЖҪәвЛщРиКұјд/min | |
CO2 | H2 | CO | |||
1 | 800 | 4 | 2.5 | 1.5 | 5 |
2 | 830 | 2 | 2 | 1 | 3 |
3 | 830 | 2 | 2 | 1 | 1 |

ЈЁ1Ј©КөСй1ЦРЈ¬ЖҪәвіЈКэK=0.9Ј»vЈЁH2Ј©=0.15mol/ЈЁLminЈ©ёГ·ҙУҰөДХэ·ҙУҰОӘ______ЈЁМоЎ°ОьЎұ»тЎ°·ЕЎұЈ©ИИ·ҙУҰЈ»
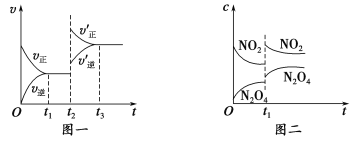
ЈЁ2Ј©КөСй3ёъКөСй2ПаұИЈ¬ёДұдөДМхјюҝЙДЬКЗ___________________________
ЈЁҙрТ»ЦЦЗйҝцјҙҝЙЈ©Ј»ИфёГ·ҙУҰ·ыәПНјЛщКҫөД№ШПөЈ¬ФтФЪНјЦРYЦбұнКҫ___________________________
ЈЁ3Ј©ДЬЕР¶ПёГ·ҙУҰҙпөҪ»ҜС§ЖҪәвЧҙМ¬өДТАҫЭКЗ______ЈЁ¶аСЎҝЫ·ЦЈ©Ј®
aЈ®ИЭЖчЦРС№ЗҝІ»ұд bЈ®»мәПЖшМеЦРcЈЁCO Ј©І»ұд
cЈ®vЈЁH2Ј©Хэ=vЈЁH2OЈ©Дж dЈ®cЈЁCO2Ј©=cЈЁCOЈ©
ЈЁ4Ј©ДіОВ¶ИПВЈ¬ЖҪәвЕЁ¶И·ыәППВКҪЈәcЈЁCO2Ј©cЈЁH2Ј©=cЈЁCOЈ©cЈЁH2OЈ©УЙҙЛҝЙТФЕР¶ПҙЛКұөДОВ¶ИОӘ______Ј®ЖдЛьМхјюІ»ұдЈ¬ЙэёЯОВ¶ИЈ¬Фӯ»ҜС§ЖҪәвПт______·ҙУҰ·ҪПтТЖ¶ҜЈЁМоЎ°ХэЎұ»тЎ°ДжЎұЈ©Ј¬ИЭЖчДЪ»мәПЖшМеөДГЬ¶И______ЈЁМоЎ°ФцҙуЎұЎўЎ°јхРЎЎұ»тЎ°І»ұдЎұЈ©Ј®
ЈЁ5Ј©