��Ŀ����
����Ŀ���ش��������⣺
(1)��̬̼ԭ�ӵĺ�������Ų�ʽΪ______���ǽ���Ԫ��![]() �ĵ�һ�����ܴ���
�ĵ�һ�����ܴ���![]() �ĵ�һ�����ܣ�ԭ����______��
�ĵ�һ�����ܣ�ԭ����______��
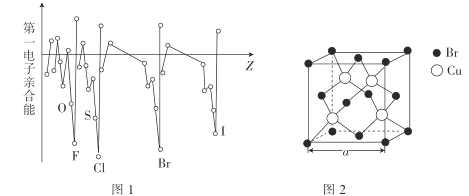
(2)�±��ǵ������ڲ���Ԫ�صĵ�����[��λ��[![]() (���ӷ���)]���ݡ�
(���ӷ���)]���ݡ�
Ԫ�� |
|
|
|
�� | 5.7 | 47.4 | 71.8 |
�� | 7.7 | 15.1 | 80.3 |
�� | 13.0 | 23.9 | 40.0 |
�� | 15.7 | 27.6 | 40.7 |
����˵����ȷ����______(�����)��
A.�Ľ����Ա���ǿ
B.����![]() ��
��
C.��������Ϊ�ǽ���Ԫ��
D.��һ��Ϊ����Ԫ��
(3)![]() ��
��![]() ��Ϊ�������ڹ��ɽ���Ԫ�أ���Ԫ�صIJ��ֵ��������������±���
��Ϊ�������ڹ��ɽ���Ԫ�أ���Ԫ�صIJ��ֵ��������������±���
Ԫ�� |
|
| |
������/( | I1 | 717 | 759 |
I2 | 1509 | 1561 | |
I3 | 3248 | 2957 | |
��Ԫ��λ�ڵ������ڵڢ�B�塣��д����̬![]() �ļ۵����Ų�ʽ��______���Ƚ���Ԫ�ص�I2��I3��֪����̬
�ļ۵����Ų�ʽ��______���Ƚ���Ԫ�ص�I2��I3��֪����̬![]() ��ʧȥ1�����ӱ���̬
��ʧȥ1�����ӱ���̬![]() ��ʧȥ1�������ѣ��Դ���Ľ�����______��
��ʧȥ1�������ѣ��Դ���Ľ�����______��
(4)±��Ԫ��![]() ��
��![]() ��
��![]() ��
��![]() �ĵ縺����С�����˳����______��
�ĵ縺����С�����˳����______��
(5)��̬![]() ԭ�ӵĵ����Ų�ʽΪ______��
ԭ�ӵĵ����Ų�ʽΪ______��![]() ��
��![]() ��ȣ��縺�Խϴ����______��
��ȣ��縺�Խϴ����______��![]() ��
��![]() Ԫ�صĻ��ϼ�Ϊ______��
Ԫ�صĻ��ϼ�Ϊ______��
���𰸡�![]()
![]() ԭ�ӵ�
ԭ�ӵ�![]() ����ﵽ�����״̬���Ƚ��ȶ� A
����ﵽ�����״̬���Ƚ��ȶ� A ![]() ��
��![]() ת��Ϊ
ת��Ϊ![]() ʱ��
ʱ��![]() �ܼ��ɽ��ȶ���
�ܼ��ɽ��ȶ���![]() �����״̬ת��Ϊ���ȶ���
�����״̬ת��Ϊ���ȶ���![]() ״̬��Ҫ�������϶ࣻ��
״̬��Ҫ�������϶ࣻ��![]() ת��Ϊ
ת��Ϊ![]() ʱ��
ʱ��![]() �ܼ��ɲ��ȶ���
�ܼ��ɲ��ȶ���![]() ״̬ת��Ϊ���ȶ���
״̬ת��Ϊ���ȶ���![]() �����״̬��Ҫ���������Ҫ��
�����״̬��Ҫ���������Ҫ�� ![]()
![]()
![]()
![]()
��������
��1��C��ԭ������Ϊ6�����̬ԭ�ӵĺ�������Ų�ʽΪ1s22s22p2��Oԭ�Ӻ�Nԭ�ӵ���Χ�����Ų�ʽ�ֱ�Ϊ2s22p4��2s22p3�����ѿ���Nԭ�ӵ�2p������ڰ����״̬���Ƚ��ȶ����ʵ�һ�����ܽϴʴ�Ϊ��1s22s22p2��![]() ԭ�ӵ�
ԭ�ӵ�![]() ����ﵽ�����״̬���Ƚ��ȶ���
����ﵽ�����״̬���Ƚ��ȶ���
��2���ס��ҡ�������Ϊ��������Ԫ�أ���Ԫ�صĵ�һ������ԶԶС�ڵڶ������ܣ�˵����Ԫ���������1�����ӣ�ʧȥ1������ʱ�ﵽ�ȶ��ṹ�����Լ�ΪNaԪ�أ���Ԫ�صĵڶ�������ԶԶС�ڵ��������ܣ���BԪ���������2�����ӣ�ʧȥ�������Ӻ�ﵽ�ȶ��ṹ��������ΪMgԪ�أ�������Ԫ�صĵ�һ���롢�ڶ������ܡ���������������˵����Ԫ����������3�����ӣ�������Ϊ�ǽ���Ԫ�أ���һ��Ϊ�ǽ���Ԫ�أ���������������֪��A����ΪNaԪ�أ���ΪMgԪ�أ��ʼĽ����Ա���ǿ����A��ȷ��B����ΪMgԪ�أ����ϼ�Ϊ+2�ۣ���B����C. ��ΪMgԪ�أ�������Ԫ�صĵ�һ���롢�ڶ������ܡ���������������˵����Ԫ����������3�����ӣ�������Ϊ�ǽ���Ԫ�أ���C����D. ������Ԫ�صĵ�һ���롢�ڶ������ܡ���������������˵����Ԫ����������3�����ӣ���һ��Ϊ�ǽ���Ԫ�أ�D����ѡA��
��3����Ԫ��λ�ڵ������ڵڢ�B�壬���̬ԭ�ӵļ۵����Ų�ʽΪ��3d54s2�������̬![]() �ļ۵����Ų�ʽ��3d5����
�ļ۵����Ų�ʽ��3d5����![]() ת��Ϊ
ת��Ϊ![]() ʱ��
ʱ��![]() �ܼ��ɽ��ȶ���
�ܼ��ɽ��ȶ���![]() �����״̬ת��Ϊ���ȶ���
�����״̬ת��Ϊ���ȶ���![]() ״̬��Ҫ�������϶ࣻ��
״̬��Ҫ�������϶ࣻ��![]() ת��Ϊ
ת��Ϊ![]() ʱ��
ʱ��![]() �ܼ��ɲ��ȶ���
�ܼ��ɲ��ȶ���![]() ״̬ת��Ϊ���ȶ���
״̬ת��Ϊ���ȶ���![]() �����״̬��Ҫ���������Ҫ�٣�����̬
�����״̬��Ҫ���������Ҫ�٣�����̬![]() ��ʧȥ1�����ӱ���̬
��ʧȥ1�����ӱ���̬![]() ��ʧȥ1�������ѣ�
��ʧȥ1�������ѣ�
��4�����ݵ縺�Եı仯���ɿ�֪��±��Ԫ��![]() ��
��![]() ��
��![]() ��
��![]() �ĵ縺����С�����˳����
�ĵ縺����С�����˳����![]() ��
��
��5����̬![]() ԭ�ӵĵ����Ų�ʽΪ��
ԭ�ӵĵ����Ų�ʽΪ��![]() ��ͬһ���ڴ�������Ԫ��ԭ�ӵĵ縺�����ʵ縺��N��B��BΪ�ڶ����ڢ�A��Ԫ�أ����̬ԭ�ӵĵ����Ų�ʽΪ��1s22s22p1��BN��N��-3�ۣ���B��+3�ۣ��ʴ�Ϊ��
��ͬһ���ڴ�������Ԫ��ԭ�ӵĵ縺�����ʵ縺��N��B��BΪ�ڶ����ڢ�A��Ԫ�أ����̬ԭ�ӵĵ����Ų�ʽΪ��1s22s22p1��BN��N��-3�ۣ���B��+3�ۣ��ʴ�Ϊ��![]() ��N��+3��
��N��+3��

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�����Ŀ���±���Ԫ�����ڱ���һ���֣����е���ĸ�ֱ����һ�ֻ�ѧԪ�ء�

(1)����Ԫ���У�ԭ����δ�ɶԵ����������� ___________ (����ĸ)��д����Ԫ�ػ�̬ԭ�ӵĺ�������Ų�ʽ�� ___________ ��
(2)�����±����ṩ�ĵ��������ݣ��ش��������⡣
� | X | Y | |
I1 | 520 | 496 | 580 |
I2 | 7296 | 4562 | 1820 |
I3 | 11799 | 6912 | 2750 |
I4 | 9543 | 11600 |
�ٱ���X����Ϊ����13��Ԫ���е� _____________ (����ĸ)Ԫ�ء���Ԫ�ط��ű�ʾX��j�γɵ�һ�ֻ�����Ļ�ѧʽ�� _____________ ��
��Y�����ڱ��е� _____________ ��Ԫ�ء�
����Ŀ��I�������£�HNO2���뷴Ӧ��ƽ�ⳣ��ֵΪ2.6��10-4��NaNO2��һ����Ҫ��ʳƷ���Ӽ�����������ۼ�ζ������ʳ�ηdz����ƣ���ʳ��ҵ�������ʳ���ж����¼�ʱ�з�����
��1��ij�С��ͬѧ���ʵ�鷽������ NaCl��Һ��NaNO2��Һ������д���б���
ѡ��ҩƷ | ʵ������ | ����NaNO2������ |
�ٷ�̪��Һ | ____________ | ____________ |
�ڵ���-KI��ֽ | ____________ | ____________ |
��2�����������ж������������ŷţ�ʵ����һ�㽫���뱥���Ȼ����Һ����ʹ֮ת���������������ʣ������֮һΪ��ɫ��ζ���壬�������Ϊ____________���ѧʽ����
II���С��ͬѧ��������װ���Ʊ����ⶨ���ù������������ƣ�NaNO2��������������װ�ÿ��ظ�ʹ�ã����ּг�������ʡ�ԣ���
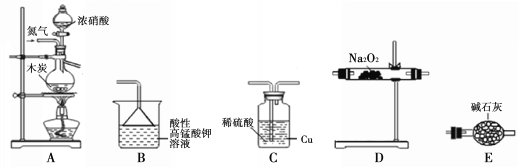
��֪�� ��2NO + Na2O2 ��2NaNO2��
�����������£�NO��NO2������MnO4����Ӧ����NO3����Mn2+��NaNO2��ʹ
���Ը��������Һ��ɫ��
��1��ʵ��װ�õ�����˳��Ϊ____________��
��2��Cƿ�ڷ�����Ӧ�����ӷ���ʽΪ____________��
��3��Ϊ�˲ⶨ�������Ƶĺ�������ȡ4.0g��Ʒ����ˮ���250mL��Һ��ȡ25.00mL��Һ����ƿ�У���0.10 mol��L��1������KMnO4��Һ���еζ���ʵ�������������±���
����� | 1 | 2 | 3 | 4 |
KMnO4��Һ���/mL | 20.60 | 20.02 | 20.00 | 19.98 |
�ٵ�һ��ʵ�����ݳ��ֽ������쳣������쳣��ԭ�������__________������ĸ��ţ���
a����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ
b����ƿ������ˮϴ����δ����
c���۲�ζ��յ�ʱ���Ӷ���
�ڸ��ݱ������ݽ��м��㣬���ƵõĹ������������Ƶ���������Ϊ____________��
��4�����ʵ�飬�Ƚ�0.1mol��L��1NaNO2��Һ��NO2����ˮ��̶Ⱥ�0.1mol��L��1HNO2��Һ��HNO2����̶ȵ���Դ�С_______����Ҫ˵��ʵ�鲽�衢����ͽ��ۣ�������ҩƷ��ѡ����