��Ŀ����
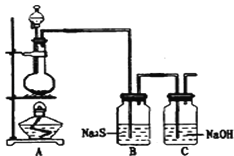
����Ŀ��ʵ����������װ����ȡ������������������ʵ�顣�ش��������⣺

��1��A��ʢ��Ũ���ᣬB��ʢ��MnO2��д����Ӧ�Ļ�ѧ����ʽ_____________��
��2��D�з���ŨH2SO4����Ŀ����_____________________________��
��3��E��Ϊ��ɫ�ɲ�����F��Ϊ��ɫʪ�������ɹ۲쵽��������___________���Ա�E��F������IJ���ɵó��Ľ��ۼ�������________________________________��
��4��G����������____________________________________��
��5������H��β������װ��ͼ��ע���Լ�____________��
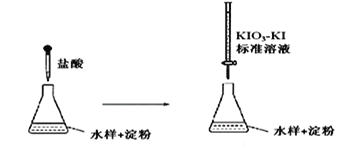
��6����ͥ�г�������Һ����Ҫ�ɷ�NaClO�������飨��Ҫ�ɷ����ᣩ���������ijƷ������Һ��װ��˵������ͼ��

�١�������ͬʱʹ�á�������ж���������д����Ӧ�����ӷ���ʽ__________��
���衰�ܱձ��桱��ԭ��____________________________________________��
��7��������һ����Ϊ����ˮ�衱�IJ�ƷҲ�ܶ�����ˮ���п��ٵ�ɱ��������ҩ��ͨ�����������㡣�������Ⱦ�Cl2Na(NCO)3����ˮ��Ӧ�����ɴ�������ɱ���������ã������Ӻ��ڲ���������ƣ�Na2SO3���ܳ����ɽ�ˮ�е����ȣ�������ȣ���ȥ���������ƽ�ˮ�ж���������ȥ�����ӷ�Ӧ����ʽΪ______________��
���𰸡�MnO2��4HCl(Ũ��![]() MnCl2��Cl2����2H2O��ȥ�����е�ˮ����E�в���ɫ��F����ɫ���������û��Ư���ԣ�������ˮ��Ӧ���ɵĴ�������Ư���Գ�����ɫ
MnCl2��Cl2����2H2O��ȥ�����е�ˮ����E�в���ɫ��F����ɫ���������û��Ư���ԣ�������ˮ��Ӧ���ɵĴ�������Ư���Գ�����ɫ Cl-+ClO-+2H+��Cl2��+H2O��������������еĶ�����̼��ӦSO32-+HClO=SO42-+H++Cl-
Cl-+ClO-+2H+��Cl2��+H2O��������������еĶ�����̼��ӦSO32-+HClO=SO42-+H++Cl-
��������
ʵ������Ũ������������̼��ȷ�Ӧ�Ʊ�������Ũ������лӷ��ԣ��Ʊ��������к����Ȼ��⡢ˮ������ͨ��ʢ�б���ʳ��ˮ��Ũ�����ϴ��ƿ��ȥ���ʣ�����ͨ��������ɫ������ʪ����ɫ������֤�����Ƿ����Ư���ԣ���ͨ��Gװ����֤�����������ԣ������ж����ܹ�������������Һ��Ӧ������������������Һ����β�����ݴ˽��
��1��MnO2��Ũ�����ڼ��������·���������ԭ��Ӧ�����������Ȼ��̺�ˮ������ʽΪMnO2��4HCl(Ũ��![]() MnCl2��Cl2����2H2O��
MnCl2��Cl2����2H2O��
��2��Ũ���������ˮ�ԣ�D��Ũ��������Ϊ������������ȥ�����е�ˮ��������ֹˮ����������Ư���Լ�����ɸ��ţ�
��3���������������ͨ��E�к�ɫ�ɲ�����F�к�ɫʪ��������������ΪE�в�������ɫ��F�в�����ɫ��˵�����������û��Ư���ԣ�������ˮ��Ӧ���ɵĴ�������Ư���ԣ�
��4����������ǿ�������ԣ���⻯�ط�Ӧ���ɵ��ʵ⣬���������۱��������Կ�������Ϊ���ű�����
��5�������ж�����ֱ���ŷŵ������У������ܹ���Ӧ������������������Һ���չ�����������װ����ͼ��ʾ ��
��
��6���ٴ�����������������ӷ���������ԭ��Ӧ����������ˮ�����ӷ���ʽΪCl-+ClO-+2H+��Cl2��+H2O��
������Һ����Ч�ɷ��Ǵ��������������̼��ˮ���ɵĴ����ᣬ������ȶ��������ֽ⣬����Ӧ���ܷⱣ�棻
��7�������������������ᷴӦ������������ӡ������ӣ���Ӧ�����ӷ���ʽΪS032-+HClO��S042-+H++Cl-��

����Ŀ��������(SO2Cl2)�����Ȼ������Ȼǻ�������������ҩƷ��Ⱦ�ϡ�������Լ��ȡ��й����ʵIJ����������±���
���� | �۵�/�� | �е�/�� | �������� |
SO2Cl2 | ��54.1 | 69.1 | ������ˮ��Ӧ�������������� ���ֽ⣺SO2Cl2 |
H2SO4 | 10.4 | 338 | ��ˮ���Ҳ��ֽ� |
ʵ�����ø���������Ķ�������������ϳ������ȣ�װ����ͼ��ʾ(�г�������ʡ��)����ش��й����⣺
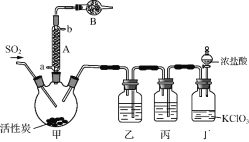
(1) ����A��ȴˮ�Ľ���_______(����a������b��)��
(2) ����B��ʢ�ŵ�ҩƷ��_______��
(3) ʵ������������������������������Ʊ����÷�Ӧ�����ӷ���ʽΪ_______����������������йص�˵������ȷ����_______��
A.��ΪSO2����Ư���ԣ���������ʹƷ����Һ����ˮ������KMnO4��Һ��ʯ����Һ��ɫ
B.��ʹƷ����Һ��ɫ�����ʲ�һ����SO2
C.SO2��Ư�ۡ�����̿��Na2O2����ʹ��īˮ��ɫ����ԭ����ͬ
D.�����ʵ�����SO2��Cl2��Ϻ�ͨ��װ��ʪ�����ɫ�����ļ���ƿ�У�Ư��Ч������
E.����Ũ�������SO2
F.���ó����ʯ��ˮ����SO2��CO2
(4) װ�ñ���ʢ�Լ�Ϊ_______����ȱ��װ���ң��������Ȼ���ʧ���÷�Ӧ�Ļ�ѧ����Ϊ______________��
(5) ����������Ҳ�����Ȼ���(ClSO3H)�ֽ��ã��÷�Ӧ�Ļ�ѧ����ʽΪ��2ClSO3H===H2SO4��SO2Cl2���˷����õ��IJ�Ʒ�л�������ᡣ
�ٴӷֽ�����з���������ȵķ�����______________��
�������ʵ�鷽�������Ʒ��������(��ѡ�Լ���ϡ���ᡢϡ���ᡢBaCl2��Һ������ˮ��ʯ����Һ)____��