��Ŀ����
����Ŀ��������(SO2Cl2)�����Ȼ������Ȼǻ�������������ҩƷ��Ⱦ�ϡ�������Լ��ȡ��й����ʵIJ����������±���
���� | �۵�/�� | �е�/�� | �������� |
SO2Cl2 | ��54.1 | 69.1 | ������ˮ��Ӧ�������������� ���ֽ⣺SO2Cl2 |
H2SO4 | 10.4 | 338 | ��ˮ���Ҳ��ֽ� |
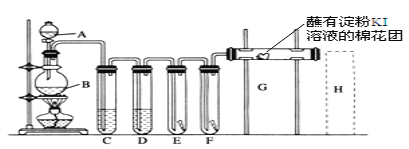
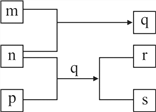
ʵ�����ø���������Ķ�������������ϳ������ȣ�װ����ͼ��ʾ(�г�������ʡ��)����ش��й����⣺

(1) ����A��ȴˮ�Ľ���_______(����a������b��)��
(2) ����B��ʢ�ŵ�ҩƷ��_______��
(3) ʵ������������������������������Ʊ����÷�Ӧ�����ӷ���ʽΪ_______����������������йص�˵������ȷ����_______��
A.��ΪSO2����Ư���ԣ���������ʹƷ����Һ����ˮ������KMnO4��Һ��ʯ����Һ��ɫ
B.��ʹƷ����Һ��ɫ�����ʲ�һ����SO2
C.SO2��Ư�ۡ�����̿��Na2O2����ʹ��īˮ��ɫ����ԭ����ͬ
D.�����ʵ�����SO2��Cl2��Ϻ�ͨ��װ��ʪ�����ɫ�����ļ���ƿ�У�Ư��Ч������
E.����Ũ�������SO2
F.���ó����ʯ��ˮ����SO2��CO2
(4) װ�ñ���ʢ�Լ�Ϊ_______����ȱ��װ���ң��������Ȼ���ʧ���÷�Ӧ�Ļ�ѧ����Ϊ______________��
(5) ����������Ҳ�����Ȼ���(ClSO3H)�ֽ��ã��÷�Ӧ�Ļ�ѧ����ʽΪ��2ClSO3H===H2SO4��SO2Cl2���˷����õ��IJ�Ʒ�л�������ᡣ
�ٴӷֽ�����з���������ȵķ�����______________��
�������ʵ�鷽�������Ʒ��������(��ѡ�Լ���ϡ���ᡢϡ���ᡢBaCl2��Һ������ˮ��ʯ����Һ)____��
���𰸡�a ��ʯ�� SO32-+2 H��=== SO2����H2O BE ����ʳ��ˮ SO2Cl2��2H2O===H2SO4��2HCl ���� ȡ�����ڸ��������¼�������ȫ��Ӧ(��ӷ���ֽ��)����ȴ���ˮϡ��,ȡ������Һ�μ���ɫʯ����Һ��죻��ȡ������Һ������BaCl2��Һ������ɫ������˵������H2SO4��
��������
��������������ϳ������ȣ���װ�ã�SO2��g��+Cl2��g��![]() SO2Cl2�������Ȼ�ˮ�⣬����B��ʢ�ŵ�ҩƷ�Ǽ�ʯ�ҷ�ֹ�����е�ˮ��������װ�ã�ͬʱ���ջӷ���ȥ�Ķ����������������װ�ã�Ũ���������ط�Ӧ��ȡ������Ũ�����ӷ�����ȡ�������к����Ȼ��⣬��װ�ã���ȥCl2�е�HCl����װ�ã�����������
SO2Cl2�������Ȼ�ˮ�⣬����B��ʢ�ŵ�ҩƷ�Ǽ�ʯ�ҷ�ֹ�����е�ˮ��������װ�ã�ͬʱ���ջӷ���ȥ�Ķ����������������װ�ã�Ũ���������ط�Ӧ��ȡ������Ũ�����ӷ�����ȡ�������к����Ȼ��⣬��װ�ã���ȥCl2�е�HCl����װ�ã�����������
��1�����ݲ�������������Ч���ã��ж������ܵĽ�ˮ�ڣ�
��2������B��ʢ�ŵ�ҩƷ�Ǽ�ʯ�ҷ�ֹ�����е�ˮ��������װ�ã�ͬʱ���ջӷ���ȥ�Ķ��������������
��3��ʵ��ʱ��װ�ö��������+5�۵��Ⱥ�������-1�۵��ȷ���������ԭ��Ӧ����������
��4�������ӷ�����ȡ�������к����Ȼ��⣬��װ�ã���ȥCl2�е�HCl��SO2Cl2��ˮ����������Ȼ��⣬����ԭ���غ���д�������������Ư���ԡ�����������ԭ�ԣ�
��5���ٶ��߷е����ϴ�ȡ�����з��룻
���Ȼ��ᣨClSO3H���ֽ⣺2ClSO3H�TH2SO4+SO2Cl2�����ʵ�鷽�������Ʒ������������ӡ����������������
��1�����ݲ�������������Ч���ã������������е���ȴˮ����Ϊa���ʴ�Ϊ��a��
��2����װ�ã�SO2��g��+Cl2��g��![]() SO2Cl2��������������Ϊ�ж����������壬���������Ȼ�ˮ�⣬��������B��ʢ�ŵ�ҩƷ�Ǽ������ʼ�ʯ�ң��ɷ�ֹ�����е�ˮ��������װ�ã�ͬʱ���ջӷ���ȥ�Ķ���������������ʴ�Ϊ����ʯ�ң�
SO2Cl2��������������Ϊ�ж����������壬���������Ȼ�ˮ�⣬��������B��ʢ�ŵ�ҩƷ�Ǽ������ʼ�ʯ�ң��ɷ�ֹ�����е�ˮ��������װ�ã�ͬʱ���ջӷ���ȥ�Ķ���������������ʴ�Ϊ����ʯ�ң�
��3�������������������Ʊ��������÷�Ӧ�����ӷ���ʽΪ��SO32-+2H+=SO2��+H2O��
A����ΪSO2����Ư���ԣ�����ʹƷ����Һ��ɫ������ˮ������KMnO4��Һ����������ԭ��Ӧ����ˮ��Ӧ����������ʹʯ����Һ��죬ѡ��A����
B����ʹƷ����Һ��ɫ�����ʿ�����SO2���������ȣ�ѡ��B��ȷ��
C��SO2��Ư�ۡ�����̿��Na2O2����ʹ��īˮ��ɫ��ԭ���ֱ�Ϊ���Ϸ�Ӧ��ǿ�����ԡ������ԡ�ǿ�����ԣ�ѡ��C����
D�������ʵ�����SO2��Cl2��Ϻ�������������ᣬ������Ư��Ч������D����
E�����߲���Ӧ������Ũ�������SO2��ѡ��E��ȷ��
F�����߾���ʯ��ˮ��Ӧ���ɰ�ɫ�����������ó����ʯ��ˮ����SO2��CO2��ѡ��F����
��ΪBE��
��4�������ӷ�����ȡ�������к����Ȼ��⣬���������ڱ���ʳ��ˮ��HCl������ˮ�������������ڱ���ʳ��ˮ�����ñ���NaCl��Һ��ȥCl2�е�����HCl�����Ա�װ������Ϊ����ȥCl2�е�HCl��SO2Cl2��ˮ����������Ȼ��⣬����ˮ�ⷽ��ʽΪ��SO2Cl2+2H2O�TH2SO4+2HCl��
��5���ٶ���Ϊ����Һ�壬�е����ϴ�ȡ�����з��룬�ʴ�Ϊ������
���Ȼ��ᣨClSO3H���ֽ⣺2ClSO3H=H2SO4+SO2Cl2��ȡ�����ڸ��������¼�������ȫ��Ӧ����ӷ���ֽ�ȣ�����ȴ���ˮϡ�ͣ�ȡ������Һ�μ���ɫʯ����Һ��죬֤����Һ�����ԣ���ȡ������Һ������BaCl2��Һ������ɫ������˵������H2SO4����ȡ��Ӧ��IJ���ֱ�Ӽ�BaCl2��Һ���а�ɫ������֤��������������ӣ��ٵμ���ɫʯ����Һ��죬˵������H2SO4��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����3�������Ϊ1 L�ĺ����ܱ������з�����Ӧ��SO2(g)��2NO(g)![]() 2NO2(g)��S(s)���ı�����I�ķ�Ӧ�¶ȣ�ƽ��ʱc( NO2)���¶ȵĹ�ϵ����ͼ��ʾ������˵����ȷ����
2NO2(g)��S(s)���ı�����I�ķ�Ӧ�¶ȣ�ƽ��ʱc( NO2)���¶ȵĹ�ϵ����ͼ��ʾ������˵����ȷ����

���� ��� | �¶�/K | ��ʼ���ʵ���/mol | |||
SO2 | NO | NO2 | S | ||
�� | 0.5 | 0.6 | 0 | 0 | |
�� | T1 | 0.5 | 1 | 0.5 | 1 |
�� | T2 | 0.5 | 0.2 | 1 | 1 |
A. �÷�Ӧ�Ħ�H<0
B. T1ʱ���÷�Ӧ��ƽ�ⳣ��Ϊ![]()
C. �����������������T1ʱ�ﵽƽ�⣬��ѹǿ֮��С��1:2
D. ��T2<T1���ﵽƽ��ʱ����������NO���������С��40%