��Ŀ����
18��ij�ʳ�����ij��ʯ[Ca3��PO4��2]��ȡ�ʲ��ۺ����ø���������ˮ��Ĺ���������ͼ��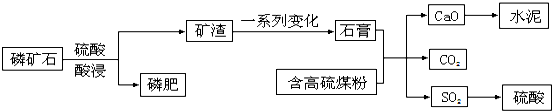
��1������ʯ�Ƴ��ʵ�Ŀ���ǽ�������ˮ��Ca3��PO4��2ת��Ϊ������ˮ��Ca��H2PO4��2������ֲ�����գ��йصĻ�ѧ����ʽΪCa3��PO4��2+2H2SO4=2CaSO4+Ca��H2PO4��2��
��2���ڸù��������и���ú�۲���Ҫ����������S��O2��Ӧ������SO2����������H2SO4��ѭ��ʹ�ã�
��3��ˮ������B���ϣ����A�����ߡ�B����A���������ǽ������� B����ͳ���ǽ������ϣ�
��4����ҵ������������У�SO2�ڽӴ����б�������ΪSO3����֪�÷�ӦΪ���ȷ�Ӧ���ֽ�2mol SO2��1mol O2�������Ϊ2L���ܱ������г�ַ�Ӧ���ų�����98.3kJ����ʱ���SO2�����ʵ���Ϊ1mol������Ȼ�ѧ����ʽΪ2SO2��g��+O2��g��?2SO3��g����H=-196.6kJ/mol��ƽ�ⳣ��KΪ4
��5����ҵ���ýӴ��������ᣬ���IJ�Ʒ��98%����������Ϊ2H2SO4•SO3�ķ������ᣨH2SO4��H2SO4•SO3�Ļ�������SO3����������ԼΪ29%������98%��Ũ����ɱ�ʾΪSO3•aH2O����SO329%�ķ�������ɱ�ʾΪbSO3•H2O����a=$\frac{10}{9}$��b=$\frac{3}{2}$��
���� ��1����ʯ������ˮ��������ֲ�����գ����ʵ���Ҫ�ɷ�Ca��H2PO4��2��������ˮ����ֲ�����գ�
��2������ú��ȼ�ղ����Ķ��������ڷ۳����������ÿɽ��������������������������
��3��ˮ�����Ҫ�ɷ��ǹ����Σ�
��4����ʱ���SO2�����ʵ���Ϊ1mol����Ӧ����1mol�Ķ�������ų�����Ϊ��98.3kJ�������Ȼ�ѧ����ʽΪ��2SO2��g��+O2��g��?2SO3��g����H=-196.6 kJ/mol����������ʽ����ƽ��ʱ����ֵ�Ũ�ȣ�Ȼ�����ƽ�ⳣ������ʽ������⣻
��5���������⣬98%����ɱ�ʾΪSO3•aH2O��ͨ��������ΪH2SO4•��a-1��H2O���ɸ���Һ��H2SO4�����ʣ���H2O���ܼ�����������ϵ�ɵã�98��18��a-1��=98%����1-98%�������a���ɣ�ͬ����29%��������ɱ�ʾΪH2O•bSO3��ͨ��������Ϊ��H2SO4•��b-1��SO3������29%����������H2SO4��SO3��������ϵ�ɵã�98��80��b-1��=��1-29%����29%�����b���ɣ�
��� �⣺��1����ʯ������ˮ��������ֲ�����գ����ʵ���Ҫ�ɷ�Ca��H2PO4��2��������ˮ����ֲ�����գ���Ӧ����ʽΪ��Ca3��PO4��2+2H2SO4=2CaSO4+Ca��H2PO4��2���ʴ�Ϊ����������ˮ��Ca3��PO4��2 ת��Ϊ������ˮ��Ca��H2PO4��2������ֲ�����գ�Ca3��PO4��2+2H2SO4=2CaSO4+Ca��H2PO4��2��
��2������ú��ȼ�ղ����Ķ��������ڷ۳����������ÿɽ��������������������������ᣬ���Կ�����Ч���ã��ʴ�Ϊ��S��O2��Ӧ������SO2����������H2SO4��ѭ��ʹ�ã�
��3��ˮ�����Ҫ�ɷ��ǹ����Σ��Ǵ�ͳ���ǽ������ϣ���ѡ��B��
��4����ʱ���SO2�����ʵ���Ϊ1mol����Ӧ����1mol�Ķ�������ų�����Ϊ��98.3kJ�������Ȼ�ѧ����ʽΪ��2SO2��g��+O2��g��?2SO3��g����H=-196.6 kJ/mol��
2SO2��g��+O2��g��?2SO3��g��
����Ũ�ȣ�1 0.5 0
�仯Ũ�ȣ�0.5 0.25 0.5
ƽ��Ũ�ȣ�0.5 0.25 0.5
����K=$\frac{0.5{\;}^{2}}{0��{5}^{2}��0.25}$=4���ʴ�Ϊ��2SO2��g��+O2��g��?2SO3��g����H=-196.6 kJ/mol��4��
��5���������⣬98%����ɱ�ʾΪSO3•aH2O��ͨ��������ΪH2SO4•��a-1��H2O���ɸ���Һ��H2SO4�����ʣ���H2O���ܼ�����������ϵ�ɵã�98��18��a-1��=98%����1-98%�������aΪ$\frac{10}{9}$��ͬ����29%��������ɱ�ʾΪH2O•bSO3��ͨ��������Ϊ��H2SO4•��b-1��SO3������29%����������H2SO4��SO3��������ϵ�ɵã�98��80��b-1��=��1-29%����29%�����b=$\frac{3}{2}$���ʴ�Ϊ��$\frac{10}{9}$��$\frac{3}{2}$��
���� ������ۺ��Ժ�ǿ���漰��֪ʶ��϶࣬��һ�����Ѷȣ��ر�ڣ�5�������ʱ��ǡ���Ķ����ݽ��д����������������ܽ�Ϊ��㣬��һ�����Ѷȣ�

| A�� | XO${\;}_{3}^{2-}$ | B�� | ZO${\;}_{4}^{2-}$ | C�� | YO${\;}_{2}^{-}$ | D�� | WO- |
��1��Z������������Ӧˮ����W����Ҫ�Ļ���ԭ�ϣ�W�Ļ�ѧʽΪH2SO4����ҵ����W������������Ҫ��Ϊ�����Σ�
��101kPaʱ��3.2g Z�Ĺ��嵥����ȫȼ�տɷų�29.7kJ��������д���ܹ���ʾ�ù��嵥��ȼ���ȵ��Ȼ�ѧ����ʽS��s��+O2��g��=SO2��g����H=-297kJ/mol��
���ڽӴ������Σ�Ϊ���ZY2��ת���ʣ����������жϣ�Ӧѡ��������ǵ��º�ѹ���ӱ�������ͬ�¶ȡ�ѹǿ��ZY2ƽ��ת���ʵ�ʵ�����ݽ��з�������Ϲ�ҵ������ʵ�ʣ�Ӧѡ���ʺϵ��¶Ⱥ�ѹǿ�ǣ�ѡ����ĸ��B��
A��400�桫500��10MPa B��400�桫500��1MPa
C��500�桫500��10MPa D��400�桫500��0.1MPa
ѹǿ/MPa ת����/% �¶�/�� | 0.1 | 0.5 | 1 | 10 |
| 400 | 99.2 | 99.6 | 99.7 | 99.9 |
| 500 | 93.5 | 96.9 | 97.8 | 99.3 |
| 600 | 73.7 | 85.8 | 89.5 | 96.4 |
A��ˮ B��0.5mol/L������ C��98.3%������ D��Ũ��ˮ
��2����֪X��XY���ǹ�ҵ�ϳ��õĻ�ԭ����
��д��X������W��Ũ��Һ��Ӧ�Ļ�ѧ����ʽC+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+2SO2��+2H2O��
��500�棬11.2L����״����ZY2�ڴ�����������XY������ѧ��Ӧ������2��6.02��1023������ת��ʱ���÷�Ӧ�Ļ�ѧ����ʽ��SO2+2CO$\frac{\underline{����}}{��}$S+2CO2��
| A�� | SO2���������� | |
| B�� | CuFeS2������ԭ������Ԫ�ر����� | |
| C�� | ÿ����1mol Cu2S����4mol������ | |
| D�� | ÿת��1.2mol���ӣ���0.2mol������ |
| A�� | 0.2mol•L-1��ˮ�е���0.2mol•L-1CH3COOH��Һ���ܴ��ڣ�c��NH4+��-��CH3COO-����c��OH-��-c��H+�� | |
| B�� | pH=5.6��NaHSO3��Һ��c��Na+����c��HSO3-����c��H2SO3����c��SO32-�� | |
| C�� | ��BaSO4����Һ�м���Na2SO4���壬Ksp��BaSO4����С | |
| D�� | ��pOH=-lgc��OH-������pH=3��������pOH=3�İ�ˮ����������Һ��PH��7 |
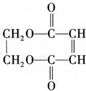 �������������ϳ���֬���ϳ��ȣ��Ա�ϩΪԭ���Ʊ�����J�ĺϳ�·����ͼ��
�������������ϳ���֬���ϳ��ȣ��Ա�ϩΪԭ���Ʊ�����J�ĺϳ�·����ͼ��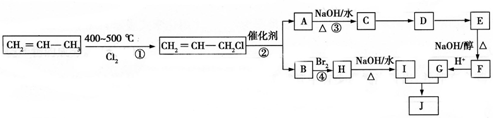
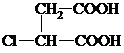 ��G�����к��еĹ��������Ȼ���̼̼˫��������������ƣ���
��G�����к��еĹ��������Ȼ���̼̼˫��������������ƣ���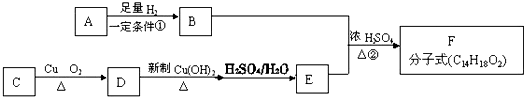
 ��
�� ��
�� ��
�� ��
��