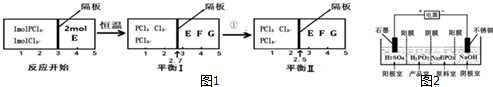
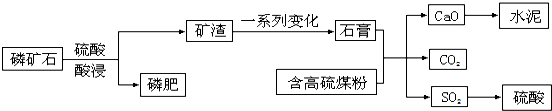
��Ŀ����
8��ij��Ԫ�� H2A �ĵ��뷽��ʽ�ǣ�H2A=H++HA-��HA-? A2-+H+���ش��������⣺��1��H2A��ǿ����ʣ��ǿ����ʡ���������ʡ��ǵ���ʡ���
��2��NaHA ��Һ�����ԣ�����ԡ��������ԡ������ԡ����������ǣ������ӷ���ʽ��ʾ��HA-?A2-+H+��
��3���� 0.1mol•L-1NaHA ��Һ�� pH=2���� 0.1mol•L-1 H2A��Һ�������ӵ����ʵ���Ũ�ȿ��ܣ�0.11mol•L�����������������=������
��4��0.1mol•L NaHA��Һ�и�����Ũ���ɴ�С��˳����c��Na+����c��HA-����c��H+����c��A2-����c��OH-����
���� ��1����H2A=H++HA-��֪��һ����ȫ���룬Ϊǿ����ʣ�
��2����һ����ȫ���룬�ڶ������ֵ��룬NaHA��Һ����Ҫ����HA-�ĵ��룬�ɵ���������ӣ�����ҺӦ�����ԣ�
��3��0.1mol•L-1H2A��Һ��H2A�TH++HA-�������0.1mol/LH+��0.1mol•L-1NaHA��Һ��pH=2������HA-?H++A2-��֪�������0.01mol/LH+������һ���������ɵ�H+������HA-�ĵ��룻
��4��NaHA��Һ�����ԣ�����HA-?H++A2-����c��Na+����c��HA-����c��H+����c��OH-�������ˮ�ĵ��������
��� �⣺��1����H2A=H++HA-��֪��һ����ȫ���룬Ϊǿ����ʣ��ʴ�Ϊ��ǿ����ʣ�
��2����һ����ȫ���룬��NaHA��Һ����Ҫ����HA-�ĵ��룬����HA-?A2-+H+����Һ�����ԣ��ʴ�Ϊ�����ԣ�HA-?A2-+H+��
��3��0.1mol•L-1H2A��Һ��H2A�TH++HA-�������0.1mol/LH+��0.1mol•L-1NaHA��Һ��pH=2������HA-?H++A2-��֪�������0.01mol/LH+������һ���������ɵ�H+������HA-�ĵ��룬������Һ�������ӵ����ʵ���Ũ��С��0.1mol/L+0.01mol/L=0.11mol•L-1��
�ʴ�Ϊ������
��4��NaHA��Һ�����ԣ�����HA-?H++A2-����c��Na+����c��HA-����c��H+����c��OH-�������ˮ�ĵ���H2O?H++OH-����c��H+����c��A2-������NaHA��Һ�и�������Ũ���ɴ�С��˳��Ϊc��Na+����c��HA-����c��H+����c��A2-����c��OH-����
�ʴ�Ϊ��c��Na+����c��HA-����c��H+����c��A2-����c��OH-����
���� ���⿼��ѧ���ε�ˮ������Լ�����Ũ��֮����غ��ϵ�ȷ����֪ʶ��Ϊ��Ƶ���㣬����ѧ���ķ��������Ŀ��飬��ȷϰ���е���Ϣ�ǽ����Ĺؼ���ע���Ԫ������������ص㣬��Ŀ�Ѷ��еȣ�

| A�� | 5�� | B�� | 6�� | C�� | 7�� | D�� | 8�� |
| Ԫ�� | A | B | C | D | E |
| ���ʻ�ṹ��Ϣ | �����µ��ʳ���̬��ԭ��������������D��ͬ | D3B���������������ӵĵ��Ӳ�ṹ��ͬ | A��C���γ����ֳ����Ļ�������ң��Ҿ��������� | ��������������ɫ���塢������ǿ���ڿ�����ȼ�����ɵ���ɫ���� | E��һ����̬���������������ڿ������ܶ�Ϊ3.0������ˮ�ɵ�ֻ����һ���ʵ���������Һ������Һ�ڷ��ù����������Ի���ǿ |
��1��B�����ڱ��е�λ���ǵڶ����ڢ�A�壬B���⻯����E���⻯��Ƚϣ��е�ϸߵ���NH3���ѧʽ����
��2��д��D3B���Ӧ������Һ�ʼ��ԣ�����ԡ��������ԡ������ԡ�����ԭ���ǣ������ӷ���ʽ��ʾ��N3-+4H2O?�T?NH3•H2O+3OH-��
��3��д�����ĵ���ʽΪ
 ����˵������ˮ��Һ�ڷ��ù����������Ի���ǿ��ԭ���û�ѧ����ʽ��ʾ��2HClO$\frac{\underline{\;\;��\;\;}}{\;}$2HCl+O2����
����˵������ˮ��Һ�ڷ��ù����������Ի���ǿ��ԭ���û�ѧ����ʽ��ʾ��2HClO$\frac{\underline{\;\;��\;\;}}{\;}$2HCl+O2������4����A��B��C��D����Ԫ���е�����Ԫ����ɵ�һ���ζ�����������Ȼ������ƣ�����ˮ��Һ�ʼ��ԣ�
I�����ü����Ȼ��Ƶ��Լ���ABC��
A�������͵��۵Ļ��Һ B��AgNO3��Һ C��ϡ����
��������ͭ˿���붡��Һ�У�û�������������������ữ����ӦѸ�ٷ�����ͭ˿�����ܽ���������ɫ��Һ��д���÷�Ӧ�����ӷ���ʽCu+2NO2-+4H+�TCu2++2NO��+2H2O��
���ݲ˺��ж���ά���غ����Σ���Ҳ���ж���һ������£�������һ������ȡ300��500mg��ʱ���ͻ������ж���ijѧϰС���õ������ⶨ�ݲ��ж��ĺ�������֪��2Na2S2O3+I2�TNa2S4O6+2NaI��ȡlkg�ݲˣ�A��B��C��D��EΪԭ������������������ֶ�����Ԫ�أ������ʻ�ṹ��Ϣ���±���
ե֭����ե����Һ���ռ�������ȡ�����������ƣ�ʹ�õ����ݲ�֭�е��ж����ζ�ת��Ϊ�����ڹ��˺����Һ�м�������������Һ���Գ�ȥɫ�أ��ٴι��˺�õ���Һ��������Һϡ����lL��ȡ25.00mL����Һ�������ϡ����͵⻯�صĻ����Һ��Ӧ����ѡ�ú��ʵ�ָʾ������Na2S2O3��Һ���еζ���������0.050mol•L-1Na2S2O3��Һ20.00mL����ش��������⣺
���о���ij�ʦ�����ݲ�ʱ�������������ij�֭���Լ��ᶡ��Σ������Ҫ����Ϊ��֭�к��зḻ��ά����C����˵��ά����C���л�ԭ�ԣ�
��ͨ�������ж�ij��һ��ʳ��0.125kg�����ݲ��Ƿ�������ж���ԭ��������345mg��300mg���������ж���
| A�� |  ����I2��NH4Cl�����ķ��� | B�� |  ��������ȡ���۲�Fe��OH��2 | ||
| C�� | 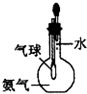 ��֤����������ˮ | D�� | 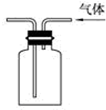 �������ſ������ռ�CO2���� |
| A�� | �ƵĻ�ѧ���ʺܻ��ã�����Ȼ���ﲻ��������̬���� | |
| B�� | ��ѧ������������ʶ���� | |
| C�� | �ڻ�ѧ��Ӧ�У��μӷ�Ӧ�ĸ����ʵ������ȵ��������ʵ���֮�� | |
| D�� | ������ѧ���Ž��з����ԭ��ѧ˵��Ϊ������ѧ�ķ�չ�춨�˼�ʵ�Ļ��� |
| A�� | Ca2+��K+��Cl-��NO3- | B�� | Ag+��Na+��NO3-��Cl- | ||
| C�� | Cu2+��K+��SO42-��Cl- | D�� | Ba2+��NH4+��SO42-��Cl- |
 ��������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣮
��������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣮| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ�Ϊ I1=738��I2=1451��I3=7733��I4=10540����λ��kJ/mol�� |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص������������������IJ�Ϊ4 |
| F��ǰ�������е縺����С��Ԫ�� |
| G�����ڱ��ĵ����� |
��2��B��̬ԭ����������ߵĵ��ӣ���������ڿռ���3������ԭ�ӹ���ʷĴ����Σ�
��3��ijͬѧ����������Ϣ���ƶ�C��̬ԭ�ӵĺ�������Ų�Ϊ��
 ��ͬѧ�����ĵ����Ų�ͼΥ��������ԭ����
��ͬѧ�����ĵ����Ų�ͼΥ��������ԭ������4��Gλ�ڵڢ�B��d�����۵����Ų�ʽΪ3d54s2��
��5��DE3����ԭ�ӵ��ӻ���ʽΪsp3���ü۲���ӶԻ��������Ʋ���ռ乹��Ϊ�����Σ�
��6��FԪ�صľ�����ͼ��ʾ������þ������ܶ�Ϊa g/cm3�������ӵ�����ΪNA��Fԭ�ӵ�Ħ������ΪM g/mol����Fԭ�ӵİ뾶Ϊ$\frac{\sqrt{3}}{4}$��$\root{3}{\frac{2M}{a{N}_{A}}}$ cm��