题目内容
【题目】Ⅰ、含苯酚的工业废水的处理流程如图所示。
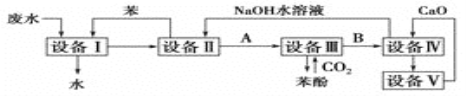
(1)①流程图设备Ⅰ中进行的是____操作(填写操作名称)。实验室里这一步操作可以用_______(填仪器名称)进行。
②向设备Ⅱ加入NaOH溶液目的是________(填化学方程式),由设备Ⅱ进入设备Ⅲ的物质A是________(填化学式),由设备Ⅲ进入设备Ⅳ的物质B是________。
③在设备Ⅲ中发生反应的化学方程式为___________________________________。
④在设备Ⅳ中,CaO与水反应后的产物与B的水溶液反应的化学方程式为_____________。通过________(填操作名称)操作,可以使产物相互分离。
⑤图中,能循环使用的物质是________、________、________、和________。
(2)为了防止水源污染,用简单而又现象明显的方法检验某工厂排放的污水中有无苯酚,此方法是_______。
(3)从溶有乙醇的苯酚溶液中回收苯酚有下列操作①蒸馏②过滤③静置分液④加入足量的金属钠⑤通入过量的CO2气体⑥加入足量的NaOH溶液⑦加入足量的FeCl3溶液 ⑧加入硫酸与NaBr共热,合理的步骤是___________
Ⅱ.某化学兴趣小组的同学在乙醛溶液中加入溴水,溴水褪色。分析乙醛的结构和性质,同学们认为溴水褪色的原因有三种可能(请补充完整):
①溴在不饱和键上发生加成反应。
②溴与甲基上的氢原子发生取代反应。
③______________________。
为确定此反应的机理,同学们进行了如下探究:
(1)向反应后的溶液中加入硝酸银溶液,若有沉淀产生,则上述第________种可能被排除。
(2)有同学提出通过检测反应后溶液的酸碱性作进一步验证,就可确定该反应究竟是何种反应原理。此方案是否可行?________,理由是__________________________。
(3)若反应物Br2与生成物Br-的物质的量之比是1∶2,则乙醛与溴水反应的化学方程式为________________________。
(4)已知烯烃能发生如下反应: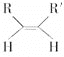
![]() RCHO+R′CHO请写出下列反应产物的结构简式:
RCHO+R′CHO请写出下列反应产物的结构简式:
![]() ___________________________________________;
___________________________________________;
【答案】萃取 分液 分液漏斗 C6H5OH+NaOH→C6H5ONa+H2O C6H5ONa NaHCO3 C6H5ONa+CO2+H2O→C6H5OH+NaHCO3 CaO+NaHCO3 = CaCO3 ↓+ NaOH 过滤 苯 CaO CO2 和NaOH溶液 加 FeCl3 溶液 ⑥①⑤③ 乙醛具有还原性,被溴水氧化 ① 否 因为第②③种反应都有HBr生成 CH3CHO+Br2+H2O===CH3COOH+2HBr ![]()
【解析】
Ⅰ、(1)①工业废水与苯进入设备Ⅰ得到苯酚、苯的溶液与可以排放的无酚工业废水,说明在设备Ⅰ中进行的是萃取,利用苯与苯酚相似的结构互溶与水不溶,将苯酚从工业废水里提取出来,用分液的方法将下层的工业废水放出排放,上层的苯酚、苯混合液进入设备Ⅱ;萃取、分液必须用到的仪器名称叫分液漏斗;故答案为:萃取、分液;分液漏斗。
②盛有苯酚、苯溶液的设备Ⅱ中注入氢氧化钠溶液,此时,具有酸性的苯酚跟氢氧化钠发生反应,生成苯酚钠和水,反应方程式为:C6H5OH+NaOH→C6H5ONa+H2O,由设备Ⅱ进入设备Ⅲ的物质A是C6H5ONa,在设备Ⅱ中的液体分为两层,上层是苯层,下层是苯酚钠的水溶液,上层的苯通过管道送回设备Ⅰ中继续萃取工业废水中的苯酚,循环使用,下层的苯酚钠溶液进入设备(Ⅲ);在盛有苯酚钠溶液的设备Ⅲ中,通入过量的二氧化碳气,发生化学反应,生成苯酚和碳酸氢钠,故答案为:C6H5OH+NaOH→C6H5ONa+H2O;C6H5ONa;NaHCO3。
③依据碳酸性比苯酚的酸性强,弱酸盐与“强”酸发生的复分解反应,二氧化碳和苯酚钠反应生成苯酚,反应的方程式为C6H5ONa+CO2+H2O→C6H5OH+NaHCO3,
故答案为:C6H5ONa+CO2+H2O→C6H5OH+NaHCO3。
④盛有碳酸氢钠溶液的设备Ⅳ中,加入生石灰,生石灰与碳酸氢钠溶液里的水反应生成氢氧化钙,熟石灰和与碳酸氢钠发生复分解反应,生成沉淀,反应的化学方程式为:CaO+NaHCO3=CaCO3↓+NaOH,溶液与沉淀通过过滤分离,
故答案为:CaO+NaHCO3=CaCO3↓+NaOH;过滤。
⑤设备Ⅴ应是石灰窑,CaCO3高温分解所得的产品是氧化钙和二氧化碳,所得二氧化碳通入设备Ⅲ,反应所得氧化钙进入设备Ⅳ.在含苯酚工业废水提取苯酚的工艺流程中,苯、氧化钙、CO2、和NaOH理论上应当没有消耗,它们均可以循环使用,故答案为:苯、氧化钙、CO2和NaOH。
(2)检验污水中有无苯酚,用简单而又现象明显的方法可利用苯酚的显色反应性质,即,先取污水与试管中,向试管中滴加FeCl3溶液,若溶液呈紫色,则表明污水中含有苯酚,反之,则表明没有苯酚,故答案为:加FeCl3溶液。
(3)根据乙醇不会和NaOH溶液反应,加入NaOH溶液后,苯酚的乙醇溶液中,乙醇不会发生任何变化,但苯酚全部变成苯酚钠;乙醇的沸点是78℃,水的沸点是100℃。这样加热到78℃左右,将乙醇全部蒸馏掉,而剩下的为苯酚钠、NaOH溶液;最后通入过量的二氧化碳气体,由于碳酸酸性强于苯酚酸性,所以加入二氧化碳后,NaOH先全部转化为NaHCO3(加入过量二氧化碳),然后苯酚钠全部转化为苯酚,苯酚不溶水,与生成的NaHCO3溶液分层,静置后分液可以得到苯酚,所以正确的操作顺序为:⑥①⑤③。
Ⅱ.③乙醛具有还原性,被溴水氧化,故答案为:乙醛具有还原性,被溴水氧化。
(1)向反应后的溶液中加入硝酸银溶液,若有沉淀产生,则上述第①种可能被排除,故答案为:①。
(2)此方案不可行,理由是因为②③反应都有酸生成,故答案为:否;因为第②③种反应都有HBr生成。
(3)若反应物Br2与生成物Br-的物质的量之比是1:2,则乙醛与溴水发生氧化还原反应,反应的化学方程式为CH3CHO+Br2+H2O→CH3COOH+2HBr,故答案为:CH3CHO+Br2+H2O→CH3COOH+2HBr。
(4)根据有机信息可得双键断裂引入醛基,因此二聚环戊二烯经过题中反应后的产物为![]() ;故答案为:
;故答案为:![]() 。
。

 名题训练系列答案
名题训练系列答案 期末集结号系列答案
期末集结号系列答案【题目】A和B两种有机物可以互溶,有关性质如下:
物质 | 密度(g·cm-3) | 熔点/℃ | 沸点/℃ | 溶解性 |
A | 0.7893 | -117.3 | 78.5 | 与水以任意比混溶 |
B | 0.7137 | -116.6 | 34.5 | 不溶于水 |
(1)要除去A和B的混合物中的少量B,可采用的_______________方法可得到A。
A.蒸馏 B.重结晶 C.萃取 D.加水充分振荡,分液
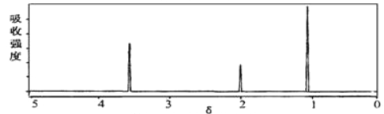
(2)将有机物A置于氧气流中充分燃烧,A和氧气恰好完全反应且消耗6.72L(标准状况)氧气,生成5.4gH2O和8.8gCO2,则该物质的实验式是__________;质谱图显示,A的相对分子质量为46,又已知有机物A的核磁共振氢谱如图所示,则A的结构简式为________________。
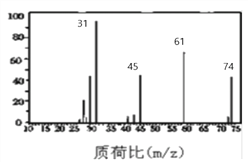
(3)下图是B的质谱图,则其相对分子质量为 ________ ,
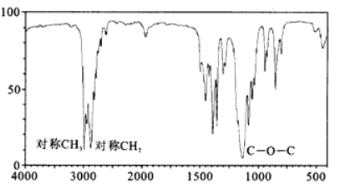
(4)B的红外光谱如图所示,则B的结构简式为__________________________。
(5)准确称取一定质量的A和B的混合物,在足量氧气充分燃烧,将产物依次通过足量的无水氯化钙和碱石灰,发现质量分别增加14.4g和26.4g。计算混合物中A和B的物质的量之比_____________________。
【题目】硫酸工业中SO2转化为SO3是重要的反应之一,在一定压强和催化剂作用下在2L密闭容器中充入0.8molSO2和2molO2发生反应:2SO2(g)+O2(g)![]() 2SO3(g),SO2的转化率随温度的变化如下表所示:
2SO3(g),SO2的转化率随温度的变化如下表所示:
温度 | 450 | 500 | 550 | 600 |
SO2的转化率% | 97.5 | 95.8 | 90.50 | 80.0 |
(1)由表中数据判断△H________0(填“>”、“=”或“<”)。
(2)能判断该反应是否达到平衡状态的是___________。
A.容器的压强不变 B.混合气体的密度不变
C.混合气体中SO3的浓度不变 D. C(SO2)=C(SO3)
E.V正(SO2)=V正(SO3) F. V正(SO3)=2V逆(O2)
(3)某温度下经2min反应达到平衡后C(SO2)=0.08mol·L-1。
①0-2min之间,O2的反应速率为____。
②此时的温度为____℃。
③此温度下的平衡常数为_____(可用分数表示)。
(4)若将平衡反应混合物的压强增大(假如体积可变),平衡将_________向移动。