ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΫΪ2molΓΛLΘ≠1AΤχΧεΚΆ1molΓΛLΘ≠1BΤχΧεΜλΚœΘ§≤Δ‘Ύ“ΜΕ®ΧθΦΰœ¬ΖΔ…ζΖ¥”ΠΘΚ2A(g)ΘΪB(g)![]() 2C(g)Θ§»τΨ≠2sΚσ≤βΒΟCΒΡ≈®Ε»ΈΣ0.6molΓΛLΘ≠1Θ§œ÷”–œ¬Ν–ΦΗ÷÷ΥΒΖ®ΘΚ
2C(g)Θ§»τΨ≠2sΚσ≤βΒΟCΒΡ≈®Ε»ΈΣ0.6molΓΛLΘ≠1Θ§œ÷”–œ¬Ν–ΦΗ÷÷ΥΒΖ®ΘΚ
ΔΌ”ΟΈο÷ B±μ ΨΒΡΖ¥”ΠΒΡΤΫΨυΥΌ¬ ΈΣ0.6molΓΛLΘ≠1ΓΛsΘ≠1
ΔΎ”ΟΈο÷ A±μ ΨΒΡΖ¥”ΠΒΡΤΫΨυΥΌ¬ ΈΣ0.3molΓΛLΘ≠1ΓΛsΘ≠1
Δέ2s ±Έο÷ AΒΡΉΣΜ·¬ ΈΣ70%
Δή2s ±Έο÷ BΒΡ≈®Ε»ΈΣ0.7molΓΛLΘ≠1
Τδ÷–’ΐ»ΖΒΡ «Θ® Θ©
A.ΔΌ ΔέB.Δέ ΔήC.ΔΎ ΔέD.ΔΎ Δή
ΓΨ¥πΑΗΓΩD
ΓΨΫβΈωΓΩ
2A(g)ΘΪB(g)![]() 2C(g)
2C(g)
≥θ Φ 2 1 0
ΗΡ±δ 0.6 0.3 0.6
2sΚσ 1.4 0.7 0.6
ΔΌ”ΟΈο÷ B±μ ΨΒΡΖ¥”ΠΒΡΤΫΨυΥΌ¬ ΈΣ![]() molΓΛLΘ≠1ΓΛsΘ≠1Θ§Ι ¥μΈσΘΜΔΎ”ΟΈο÷ A±μ ΨΒΡΖ¥”ΠΒΡΤΫΨυΥΌ¬ ΈΣ
molΓΛLΘ≠1ΓΛsΘ≠1Θ§Ι ¥μΈσΘΜΔΎ”ΟΈο÷ A±μ ΨΒΡΖ¥”ΠΒΡΤΫΨυΥΌ¬ ΈΣ![]() molΓΛLΘ≠1ΓΛsΘ≠1Θ§Ι ’ΐ»ΖΘΜΔέ2s ±Έο÷ AΒΡΉΣΜ·¬ ΈΣ
molΓΛLΘ≠1ΓΛsΘ≠1Θ§Ι ’ΐ»ΖΘΜΔέ2s ±Έο÷ AΒΡΉΣΜ·¬ ΈΣ![]() Θ§Ι ¥μΈσΘΜΔή2s ±Έο÷ BΒΡ≈®Ε»ΈΣ0.7molΓΛLΘ≠1Θ§Ι ’ΐ»ΖΓΘ
Θ§Ι ¥μΈσΘΜΔή2s ±Έο÷ BΒΡ≈®Ε»ΈΣ0.7molΓΛLΘ≠1Θ§Ι ’ΐ»ΖΓΘ
Ι ―ΓDΓΘ

ΓΨΧβΡΩΓΩ”Οœ¬ΆΦΥυ ΨΉΑ÷ΟΫχ––œ¬Ν– Β―ιΘΚΫΪΔΌ÷–»ή“ΚΒΈ»κΔΎ÷–Θ§‘Λ≤βΒΡœ÷œσ”κ ΒΦ œύΖϊΒΡ «
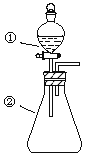
―Γœν | ΔΌ÷–Έο÷ | ΔΎ÷–Έο÷ | ‘Λ≤βΔΎ÷–ΒΡœ÷œσ |
A | œΓ―ΈΥα | ΧΦΥαΡΤ”κ«β―θΜ·ΡΤΒΡΜλΚœ»ή“Κ | ΝΔΦ¥≤ζ…ζΤχ≈ί |
B | ≈®œθΥα | ”Ο…Α÷Ϋ¥ρΡΞΙΐΒΡ¬ΝΧθ | ≤ζ…ζΚλΉΊ…ΪΤχΧε |
C | –¬÷Τ¬»Υ° | ΒμΖέΒβΜ·ΦΊ»ή“Κ | »ή“Κ±δάΕ…Ϊ |
D | ≈®―ΈΥα | MnO2 | ≤ζ…ζΜΤ¬Χ…ΪΤχΧε |
A.AB.BC.CD.D