��Ŀ����
15��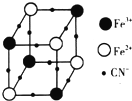 ���ɽ���Ԫ�ؼ��仯�����ںϽ�����Լ������ȷ���Ӧ�÷dz��㷺��
���ɽ���Ԫ�ؼ��仯�����ںϽ�����Լ������ȷ���Ӧ�÷dz��㷺����1����̬Niԭ�ӵļ۵��ӣ���Χ���ӣ��Ų�ʽ��ʾΪd84s2��
��2����Ϊ��4����Ԫ�أ����ڵ�Ԫ��������壬��3��Ԫ�صĵ�һ�����ܴӴ�С˳��ΪBr��As��Se����Ԫ�ط��ű�ʾ����
��3��Cu����Ķѻ���ʽ�������������ܶѻ�����ѻ����ƣ�����ͭ����������Һ�м��������ˮ��������[Cu��NH3��4]SO4������˵����ȷ����AD��
A��[Cu��NH3��4]SO4�����Ļ�ѧ�������Ӽ������Լ�����λ��
B����[Cu��NH3��4]2+��Cu2+�����µ��Ӷԣ�NH3�ṩ�չ��
C��[Cu��NH3��4]SO4���Ԫ���е縺����������Ԫ��
D��SO42-��PO43-��Ϊ�ȵ����壬����ԭ�ӵ��ӻ�������;�Ϊsp3�ӻ�
��4����һ�־���KxFey��CN��z��Fe2+��Fe3+��CN-���Ų���ͼ��ʾ��Fe2+��Fe3+λ��������Ķ��㣬�����������ڣ�CN-λ������������ϣ�ÿ��һ�������壬������������ĺ���һ��K+ ��δ������������Ļ�ѧʽ���Ա�ʾΪKFe2��CN��6��1molKxFey��CN��z���庬�Цм��ĸ���Ϊ12NA�������ӵ�������NA��ʾ����
���� ��1��Ni��28��Ԫ�أ����������Ų�ʽΪ��1s22s22p43s23p43d84s2���ݴ��ж�����Χ�����Ų�ʽ��
��2��ͬһ�����У�Ԫ�صĵ�һ����������ԭ�����������������������ƣ�����IIA��͵�VA��Ԫ�صĵ�һ�����ܴ���������Ԫ�أ�
��3��Cu����Ķѻ���ʽ�������������ܶѻ������ݵ�[Cu��NH3��4]SO4�ṹ�ж�ѡ�
��4�����þ�̯�����㣬λ�ڶ����ԭ����$\frac{1}{8}$���ڸþ�����λ�����ĵ�ԭ����$\frac{1}{2}$���ڸþ�����λ�����ϵ�ԭ����$\frac{1}{4}$���ڸþ�����λ�ھ����ڲ���ԭ��ȫ�����ڸþ�����һ�������к��������м���CN-�к���һ��������
��� �⣺��1��Ni��28��Ԫ�أ����������Ų�ʽΪ��1s22s22p43s23p43d84s2������Χ�����Ų�ʽΪ3d84s2��
�ʴ�Ϊ��3d84s2��
��2��As��Se��Br����ͬһ������ԭ������������������Ԫ���������ڵ�VA�塢��VIA�塢��VIIA�壬��VA��Ԫ�ش���������Ԫ�صĵ�һ�����ܣ�����3��Ԫ�صĵ�һ�����ܴӴ�С˳��ΪBr��As��Se��
�ʴ�Ϊ��Br��As��Se��
��3��Cu����Ķѻ���ʽ�������������ܶѻ���
A��N��Hԭ���γɼ��Թ��ۼ���[Cu��NH3��4]2+��SO42-�γ����Ӽ���Cu2+��NH3�γ���λ������A��ȷ��
B����[Cu��NH3��4]2+��Cu2+�ṩ�չ����NH3�ṩ�µ��Ӷԣ���B����
C��[Cu��NH3��4]SO4���Ԫ����OԪ�صķǽ�������ǿ����縺��������OԪ�أ���C����
D��SO42-��PO43-ԭ�Ӹ�����ȣ��۵�������ȣ���Ϊ�ȵ����壬��۲���Ӷ�����Ϊ4������sp3�ӻ�����D��ȷ��
�ʴ�Ϊ�������������ܶѻ���AD��
��4���ɾ���ͼ��֪��Fe2+��Fe3+λ��������Ķ��㣬Fe2+����ĿΪ4��$\frac{1}{8}$=$\frac{1}{2}$��Fe3+����ĿΪ4��$\frac{1}{8}$=$\frac{1}{2}$��CN-λ������������ϣ�����ĿΪ$\frac{1}{4}$��12=3��ÿ��һ�������壬������������ĺ���һ��K+����������������һ��K+����þ����к���$\frac{1}{2}$��K+����ѧʽ��дΪ��KFe2��CN��6��һ�������к��������м���CN-�к���һ��������ÿ�������к���6��CN-��������12���м�����1molKxFey��CN��z���庬�Цм��ĸ���Ϊ12NA��
�ʴ�Ϊ��KFe2��CN��6��12NA��
���� ���⿼���˾����ļ��㡢Ԫ�������ɡ������Ų�ʽ����һ�����ܡ�ԭ���ӻ���֪ʶ�㣬��Щ����ѧϰ�ص�Ҳ��ѧϰ�ѵ㣬ͬʱ����ѧ���Ŀռ���������������������������ѧ�����������ȣ��ѶȽϴ�

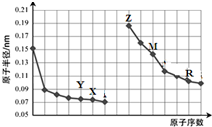
| A�� | �����ӵİ뾶��С�Ƚϣ�Y��X��Z | |
| B�� | ��̬�⻯���ȶ��ԣ�X��R | |
| C�� | R����������Ӧˮ�����м������Ӽ����й��ۼ� | |
| D�� | Z�����ܴ�M������Һ���û�������M |
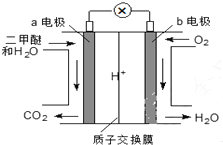 �����ѣ�CH3OCH3����һ����ɫ��������������Դ����ͼ����ɫ��Դ��ȼ�ϵ�ء��Ĺ���ԭ��ʾ��ͼ��a��b��Ϊ���Pt�缫�����õ�ع���ʱ������˵������ȷ���ǣ�������
�����ѣ�CH3OCH3����һ����ɫ��������������Դ����ͼ����ɫ��Դ��ȼ�ϵ�ء��Ĺ���ԭ��ʾ��ͼ��a��b��Ϊ���Pt�缫�����õ�ع���ʱ������˵������ȷ���ǣ�������| A�� | a�缫Ϊ�õ�ظ��� | |
| B�� | O2��b�缫�Ϸ�ӦΪO2+2H2O+4e-=4OH- | |
| C�� | ��ع���ʱ����a�缫����1molCH3OCH3�ŵ磬��·����12mol����ת�� | |
| D�� | ��ع���ʱ������ڲ�H+��a�缫����b�缫 |
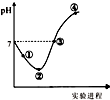 �����£���Cl2����ͨ��һ������ˮ�������ͣ�Ȼ�������ñ�����ˮ����μ���0.1mol•L-1������������Һ������������pH�仯��ͼ��ʾ�������й�������ȷ���ǣ�������
�����£���Cl2����ͨ��һ������ˮ�������ͣ�Ȼ�������ñ�����ˮ����μ���0.1mol•L-1������������Һ������������pH�仯��ͼ��ʾ�������й�������ȷ���ǣ�������| A�� | ������㴦ˮ�ĵ���̶������pH��ֽ�ⶨ��ҺpH | |
| B�� | �ڵ�֮ǰ��������Ӧ�����ӷ���ʽΪCl2+H2O=2H++Cl-+ClO- | |
| C�� | ��������ˮ��ϵ�У�c��H+��=c��Cl2��+c��HClO��+c��ClO-�� | |
| D�� | �۵���ʾ��Һ�У�c��Na+��=c��Cl-��+c��ClO-�� |

| A�� | �ŵ�ʱ��OH-�����缫�������ƶ� | |
| B�� | �ŵ�ʱ�������ĵ缫��ӦΪ��H2-2e-+2OH-�T2H2O | |
| C�� | ���ʱ������ص�̼�缫�����Դ���������� | |
| D�� | ���ʱ�������ĵ缫��ӦΪ��Ni��OH��2+OH--e-�TNiO��OH��+H2O |
| X | Y | ||
| Z | W |
| A�� | Xλ��Ԫ�����ڱ��е�2���ڡ��ڢ�A�� | |
| B�� | Y��һ���⻯�ﲻ�ȶ����ֽ� | |
| C�� | W�ķǽ����Ա�Y�ķǽ������� | |
| D�� | Z������������ˮ�������X������������ˮ���ﷴӦ |