��Ŀ����
13��A��B��C��D���ֶ���������Ԫ�ص�ԭ���������μ�С��D�ڶ���������Ԫ����ԭ�Ӱ뾶���Cԭ����������������Ӳ�����ȣ�BԪ�ص���Ҫ���ϼۣ��������+�����=4������˵����ȷ���ǣ�������| A�� | C���������Ǽ��������� | |
| B�� | D��A���γ�ԭ�Ӹ�����Ϊ1��1�͵����ӻ����� | |
| C�� | ����������Ӧˮ��������ԣ�B��A | |
| D�� | ��ҵ�ϵ����A��C����Ԫ����ɵĻ������Ʊ�C���� |
���� A��B��C��D���ֶ���������Ԫ�ص�ԭ���������μ�С��D�ڶ���������Ԫ����ԭ�Ӱ뾶�����DΪNa��BԪ�ص���Ҫ���ϼۣ��������+�����=4�����ڢ�A�壬��BΪSԪ�أ�Cԭ����������Na��С��S����C���ڵ������ڣ���Cԭ����������������Ӳ�����ȣ���CΪAl��A��ԭ������������AΪCl���ݴ˽��
��� �⣺A��B��C��D���ֶ���������Ԫ�ص�ԭ���������μ�С��D�ڶ���������Ԫ����ԭ�Ӱ뾶�����DΪNa��BԪ�ص���Ҫ���ϼۣ��������+�����=4�����ڢ�A�壬��BΪSԪ�أ�Cԭ����������Na��С��S����C���ڵ������ڣ���Cԭ����������������Ӳ�����ȣ���CΪAl��A��ԭ������������AΪCl��
A��C�������������������������������A����
B��D��A���γ�NaCl���������ӻ������B��ȷ��
C�����ڷǽ�����A��Cl����B��S����������������Ӧˮ��������ԣ���������ᣬ��C����
D���Ȼ������ڹ��ۻ���������磬��ҵ�ϵ�������������Ʊ�Al���ʣ���D����
��ѡB��
���� ���⿼��λ�ýṹ���ʹ�ϵӦ�ã��ƶ�Ԫ���ǽ���ؼ���ע��Dѡ��������ұ����������ѧ�����Ļ�ѧ��ҵ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| A�� | װ�üף�ʯ�ͷ��� | B�� | װ���ң��屽����ȡ | ||
| C�� | װ�ñ�����ϩ����ȡ | D�� | װ�ö���������������ȡ |
| A�� | ��pH=5�Ĵ�����Һϡ�ͺָ���ԭ�¶ȣ�pH��Kw������ | |
| B�� | 25��ʱ��pH=3���Ȼ����Һ�У�c��OH-��=1.0��10-11mol•L-1 | |
| C�� | 25��ʱ��pH=4���Ȼ����Һ�У�c��Cl-����c��NH4+����c��H+����c��OH-�� | |
| D�� | ��NH4HSO4��Һ�м�������ʵ�����NaOH�γɵ���Һ�У�c��Na+��=c��SO42-����c��NH4+����c��H+����c��OH-�� |
| A�� | KMnO4 | B�� | KClO3 | C�� | MnO2 | D�� | Ca��ClO��2 |
| A�� | ԭ�Ӱ뾶 Na��Mg��Al | B�� | ���� H2SiO3��H2CO3��H2SO4 | ||
| C�� | �ȶ��� HF��HCl��HBr | D�� | ���� NaOH��Mg��OH��2��Al��OH��3 |
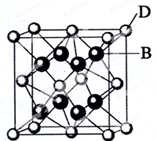 ��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36��Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壮B��C��ͬһ���壬D��E��ͬһ���ڣ�D��ԭ��������EС5����֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D�ļ۵�����Ϊ2��D��B���γ����ӻ�����侧���ṹ��ͼ����ش�
��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36��Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壮B��C��ͬһ���壬D��E��ͬһ���ڣ�D��ԭ��������EС5����֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D�ļ۵�����Ϊ2��D��B���γ����ӻ�����侧���ṹ��ͼ����ش� 50mL 0.50mol•L-1������50mL 0.55mol•L-1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����кͷ�Ӧ�ķ�Ӧ�ȣ�
50mL 0.50mol•L-1������50mL 0.55mol•L-1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����кͷ�Ӧ�ķ�Ӧ�ȣ�