��Ŀ����
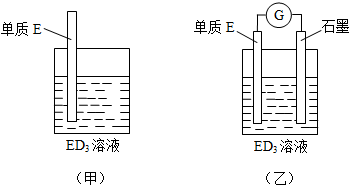
7�� 50mL 0.50mol•L-1������50mL 0.55mol•L-1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����кͷ�Ӧ�ķ�Ӧ�ȣ�
50mL 0.50mol•L-1������50mL 0.55mol•L-1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����кͷ�Ӧ�ķ�Ӧ�ȣ��ش��������⣺
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ�ǻ��β��������
��2���ձ�����������ĭ���ϵ������Ǽ���ʵ������е�������ʧ
��3�����ձ����粻��Ӳֽ�壬��õ��кͷ�Ӧ�ķ�Ӧ�ȵ�
��ֵƫС���ƫ��ƫС������Ӱ�족����
��4��ʵ���и���60mL 0.50mol•L-1�����50mL 0.55mol•L-1NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�����������ȣ����ȡ�����ȡ�����
��5������ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬��õ��кͷ�Ӧ�ķ�Ӧ�Ȼ�ƫС���ƫ��ƫС������Ӱ�족��
���� ��1���������ȼƵĹ������жϸ�װ�õ�ȱ��������
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�����
��3�����ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ��
��4����Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��5������������ʵ������ȷ�����
��� �⣺��1�������ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β������������ʴ�Ϊ�����β�����������
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�������С�ձ�֮��������ֽ���������Ǽ���ʵ������е�������ʧ���ʴ�Ϊ������ʵ������е�������ʧ��
��3�����ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ����õ��к�����ֵ�����С���ʴ�Ϊ��ƫС��
��4����Ӧ�ų����������������Լ�������Ķ����йأ�������60mL0.50mol•L-1������50mL0.55mol•L-1NaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ��ʴ�Ϊ������ȣ�
��5��һˮ�ϰ�Ϊ����������Ϊ���ȹ��̣������ð�ˮ����ϡ����������Һ��Ӧ����Ӧ�ų�������С��57.3kJ���ʴ�Ϊ��ƫС��
���� ���⿼���й��к��ȵIJⶨ֪ʶ��ע���к������ᡢ������ʵ����أ����Ը�����ѧ֪ʶ���лش��ѶȲ���

| A�� | C���������Ǽ��������� | |
| B�� | D��A���γ�ԭ�Ӹ�����Ϊ1��1�͵����ӻ����� | |
| C�� | ����������Ӧˮ��������ԣ�B��A | |
| D�� | ��ҵ�ϵ����A��C����Ԫ����ɵĻ������Ʊ�C���� |
 ����NaBH4-H2O2ȼ�ϵ�أ�DBFC���Ľṹ��ͼ��ʾ����֪NaBH4����Ϊ-1�ۣ����йظõ�ص�˵������ȷ���ǣ�������
����NaBH4-H2O2ȼ�ϵ�أ�DBFC���Ľṹ��ͼ��ʾ����֪NaBH4����Ϊ-1�ۣ����йظõ�ص�˵������ȷ���ǣ�������| A�� | �ŵ������Na+��A������B����Ǩ�� | |
| B�� | �缫B�����к�MnO2�㣬MnO2������ | |
| C�� | �ڵ�ط�Ӧ�У�ÿ����1 L 6 mol•L-1H2O2��Һ��������������·�еĵ���Ϊ12 ��NA | |
| D�� | ��ظ������ĵ缫��ӦΪBH4-+8OH--8e-=BO2+6H2O |
| A�� | ���Ƿ���������һ����CH2ԭ���ŵ����ʣ��˴�һ����ͬϵ�� | |
| B�� | ���ֻ��������Ԫ����ͬ����Ԫ����������Ҳ��ͬ��������һ����ͬ���칹�� | |
| C�� | ��Է���������ͬ�ļ��ֻ��������Ϊͬ���칹�� | |
| D�� | ���Ԫ�ص�����������ͬ������Է�������Ҳ��ͬ�IJ�ͬ�����һ����Ϊͬ���칹�� |
| A�� | 1.68g���۷���������ʴʱ���������������������504mL����״���� | |
| B�� | �Ʋ������������п�����������������������������ױ���ʴ | |
| C�� | Ϊ�������ֵĴ��ǣ����ڴ�����������п�� | |
| D�� | ��ѧ��Դ��������Դ��������ԭ��Ӧ���ͷŵĻ�ѧ�� |
| A�� | ��ʢ������Mg��OH��2�������Թ��еμ�����NH4Cl��Һ�������ܽ⣺Mg��OH��2+2NH4+=2NH3•H2O+Mg2+ | |
| B�� | SO2ͨ�����ʯ��ˮ�У�������ɫ������SO2+Ca2++2OH-=CaSO4��+H2O | |
| C�� | ��Ca��HCO3��2��Һ�м������NaOH��Һ���а�ɫ�������ɣ�Ca2++2HCO3-+2OH-�TCaCO3��+CO32-+2H2O | |
| D�� | ���ڹ������ð�˾ƥ�ֳ���ˮ���ᣨ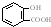 ����Ӧ���ɾ���ע��NaHCO3��Һ�� ����Ӧ���ɾ���ע��NaHCO3��Һ��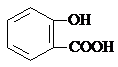 +HCO3-�� +HCO3-��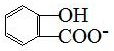 +CO2��+H2O +CO2��+H2O |

��a��b��c�������㣩����˵����ȷ���ǣ�������
| A�� | H2��I2��HI���ǹ��ۻ����� | |
| B�� | �Ͽ�2 mol HI�����еĻ�ѧ����������ԼΪ��c+b+2a�� kJ | |
| C�� | ��ͬ�����£�1 mol H2��g����1mol I2��g��������С��2 mol HI ��g���������� | |
| D�� | ���ܱ������м���1 mol H2��g����1 mol I2��g������ַ�Ӧ��ų�������Ϊ2a kJ |
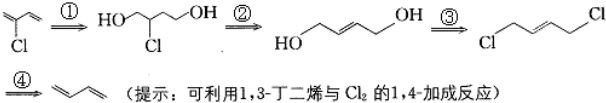
 �����Ʊ��ȶ���ԭ�ϣ���ֻ��1��3-����ϩ��
�����Ʊ��ȶ���ԭ�ϣ���ֻ��1��3-����ϩ�� ������һ����ԭ�ӣ�������˫���ϵ���ԭ�Ӻ��ѷ���ȡ����Ӧ������ͨ��1��3-����ϩֱ����������Ӧ�Ƶã�2-��-1��3-����ϩ����ϳɷ���Ϊ��
������һ����ԭ�ӣ�������˫���ϵ���ԭ�Ӻ��ѷ���ȡ����Ӧ������ͨ��1��3-����ϩֱ����������Ӧ�Ƶã�2-��-1��3-����ϩ����ϳɷ���Ϊ��