��Ŀ����
����Ŀ��������һ�������·�������ת����ϵ��

��֪��������H�к���һ��������ش�
��1��д��B�ĵ���ʽ___��������D�еĹ���������___��
��2��E+H��G�Ļ�ѧ����ʽ��___��
��3������˵������ȷ����___��
A��������A��D����������Һ��Ӧ���ɹ���������
B�������Ƶ�������ͭ�������ֻ�����C��D��E
C����ͬ������A��E��H���ȼ��ʱ���ĵ���������
D��������F��G��Ϊͬ���칹��
���𰸡�![]() ȩ��
ȩ�� ![]() +CH3COOH
+CH3COOH![]() +H2O BD
+H2O BD
��������
������ϡ�����������ˮ�����������ǡ�C������������E�����������ǵ����ʿ���֪��CӦ��Ϊ�Ҵ���BΪ������̼���Ҵ���������D��DΪ��ȩ����ȩ������E��EΪ���ᡣH�ܺ����ᡢ�Ҵ�����������Ӧ��˵��H�к����Ȼ����ǻ���������֪������H�к���һ�����ͷ���ʽ��֪��HΪ![]() ��
��
��1���������ھƻ�ø�������������Ҵ��Ͷ�����̼��CΪ�Ҵ���BΪ������̼��������̼����ʽΪ![]() ��DΪ��ȩ���������Ϊȩ����
��DΪ��ȩ���������Ϊȩ����
��2��EΪCH3COOH��HΪ![]() �����߷���������Ӧ����ѧ����ʽΪ
�����߷���������Ӧ����ѧ����ʽΪ![]() +CH3COOH
+CH3COOH![]() +H2O��
+H2O��
��3��A�������Ǻ���ȩ�к���ȩ��������������Һ����������Ӧ�����ɹ��������������������⣬A����
B��C�Ҵ���������ͭ��Һ����Ӧ������D��ȩ�������Ƶ�������ͭ��Ӧ����ש��ɫ������E��������������ͭ��Ӧ�����ܽ�������ɫ��Һ�������߿������Ƶ�������ͭ���֣�ѡ��������⣬B��ȷ��
C��A��E��H�ķ������ʽ��ΪCH2O������ͬ������A��E��H���ȼ��ʱ��������������ͬ��ѡ��������⣬C����
D��������F��G�ķ���ʽ��ͬ�����߲���ͬ���칹�壬�������⣬D��ȷ��
��ѡBD��

����Ŀ���±��Dz�ͬ�¶���ˮ�����ӻ������ݣ�
�¶� | 25 |
|
|
ˮ�����ӻ� |
| a |
|
�Իش���������
(1)��![]() ����a______1��10-14�<����>����=��)��
����a______1��10-14�<����>����=��)��
(2)250Cʱ��ijNa2SO4��Һ��c(SO42-)=5��10-4mol/L��ȡ����Һ1mL��ˮϡ����10mL����ϡ�ͺ���Һ��![]() ��
��![]() ______��
______��
(3)��![]() �¶��²��ij��Һ
�¶��²��ij��Һ![]() ������Һ��______
������Һ��______![]() ������������������������
������������������������![]() ��
��![]() �����¶���
�����¶���![]() ��NaOH��ҺaL��
��NaOH��ҺaL��![]() ��
��![]() ��ҺbL��ϣ������û��Һ
��ҺbL��ϣ������û��Һ![]() ����a��b______��
����a��b______��
(4)ijͬѧ��0.1 mol��L��1��NaOH��Һ�ֱ�ζ�20.00 mL0.1 mol��L��1��HCl��Һ��0.1 mol��L��1��CH3COOH��Һ���õ���ͼ��ʾ�������ζ����ߣ���ش��й����⣺
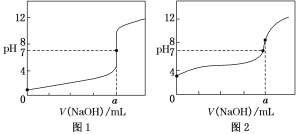
��0.1 mol��L��1��NaOH��Һ�ζ�0.1 mol��L��1��CH3COOH��Һ��������______(����ͼ1������ͼ2��)���ߡ�
��a��________��
(5)ijͬѧ��0.1 mol��L��1��NaOH��Һ�ֱ�ζ�20.00 mLδ֪Ũ�ȵ�HCl��Һѡ��_______��ָʾ������װ��Һ�ĵζ���δ��ϴ��ᵼ�²ⶨ���________������ƫ��������ƫ����������Ӱ������
����Ŀ����������Ψһ����֬���ԵĹ��ᣬ��ҽҩ��ҵ������ͷ������Ѫ������ҩ����������������ҩ������ƥĪ�ֵȵ��м��壬Ҳ������������ʵ����������ԭ���Ʊ���

�ϳɱ������ʵ�鲽�衢װ��ʾ��ͼ������������£�
���� | ״̬ | �۵�/�� | �е�/�� | �ܽ��� |
������ | ��ɫ������ | 119 | 300 | ��������ˮ�����Ѻ������ |
���� | ��ɫ��Һ�� | -116.3 | 34.6 | ���ڵ�̼���������ȷ£�����ˮ |
����ȩ | ��ɫҺ�� | -26 | 179 | ����ˮ�������Ҵ������ѡ������ȷµȻ��� |
�ȷ� | ��ɫҺ�� | -63.5 | 61.3 | �����ڴ����ѡ�����������ˮ |
ʵ�鲽�裺

����һ������ͼ��ʾ��ʵ��װ���м���0.1mol(Լl0.1mL)����ȩ��0.2mol��Լ16mL���ȷ£��������뺬19g�������Ƶ���Һ��ά���¶���55��60�棬���貢������Ӧ1h������ӦҺ��pH�ӽ�����ʱ��ֹͣ��Ӧ��
�����������ӦҺ��200mLˮϡ�ͣ�ÿ����20mL������ȡ���Σ��ϲ��Ѳ㣬�����ա�
��������ˮ����50%�������ữ��pHΪ2��3����ÿ����40mL���ѷ�������ȡ���ϲ���ȡҺ������������ˮ�����ƣ��������ѣ��ôֲ�ƷԼ11.5g����ش��������⣺
(1)ͼ������C��������_________________��װ��B��������_____________
(2)����һ�к��ʵļ��ȷ�ʽ��_____________________��
(3)������������ѵ�Ŀ����_____________________��
(4)�������м���������ˮ�����Ƶ�Ŀ����_____________________��
(5)��ʵ��IJ���Ϊ____________��������λ��Ч���֣���
(6)�������ڸ߶˻�ѧ��Ҳ�к���Ҫ�����ã����Ա�������������Ϊԭ�ϣ��ڴ����Ĵ��¿ɺϳ����Բ�ּ�����������������д���÷�Ӧ�Ļ�ѧ����ʽ___________��