��Ŀ����
��8�֣�����A��B��C��D��E����Ԫ�أ����Ƕ�����Ԫ�أ�����A��B��Cԭ���������ε����������1��B�������ӵĵ��Ӳ�ṹ��Neԭ����ͬ��2gB����������100ml 0.5mol/L�� ǡ����ȫ��Ӧ��B������D���ʷ�Ӧ����γ����ӻ�����
ǡ����ȫ��Ӧ��B������D���ʷ�Ӧ����γ����ӻ�����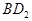 ��B������E���ʿ��γɻ�����BE��D�������ӱ�B�������Ӷ�һ�����Ӳ㣬��E��������B�������ӵ��Ӳ�ṹ��ͬ��
��B������E���ʿ��γɻ�����BE��D�������ӱ�B�������Ӷ�һ�����Ӳ㣬��E��������B�������ӵ��Ӳ�ṹ��ͬ��
(1)����Ԫ�ص�������B________��D ��E________��
(2) �γɻ����� �Ļ�ѧ�������ǣ� ��
�Ļ�ѧ�������ǣ� ��
(3)�õ���ʽ��ʾ������BD2���γɹ��̡� ��
(4) A�����������ˮ��Һ��C���ʷ�����Ӧ�����ӷ���ʽ�� �� ��
 ǡ����ȫ��Ӧ��B������D���ʷ�Ӧ����γ����ӻ�����
ǡ����ȫ��Ӧ��B������D���ʷ�Ӧ����γ����ӻ�����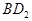 ��B������E���ʿ��γɻ�����BE��D�������ӱ�B�������Ӷ�һ�����Ӳ㣬��E��������B�������ӵ��Ӳ�ṹ��ͬ��
��B������E���ʿ��γɻ�����BE��D�������ӱ�B�������Ӷ�һ�����Ӳ㣬��E��������B�������ӵ��Ӳ�ṹ��ͬ��(1)����Ԫ�ص�������B________��D ��E________��
(2) �γɻ�����
 �Ļ�ѧ�������ǣ� ��
�Ļ�ѧ�������ǣ� ��(3)�õ���ʽ��ʾ������BD2���γɹ��̡� ��
(4) A�����������ˮ��Һ��C���ʷ�����Ӧ�����ӷ���ʽ�� �� ��
(1) B_þ��D�ȣ�E ���� ��ÿ��1�֣�
(2)���Ӽ��������ԣ����ۼ�����1�֣�ֻ��һ�ּ������֣�
(3) ��2�֣�
��2�֣�
(4)2OH-+2Al +2H2O=2AlO2-+3 H2������2�֣�
(2)���Ӽ��������ԣ����ۼ�����1�֣�ֻ��һ�ּ������֣�
(3)
 ��2�֣�
��2�֣�(4)2OH-+2Al +2H2O=2AlO2-+3 H2������2�֣�
����B������D���ʷ�Ӧ����γ����ӻ�����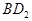 ��˵��B�ǵڢ�A��D�ǵ���A��2gB����������100ml 0.5mol/L��
��˵��B�ǵڢ�A��D�ǵ���A��2gB����������100ml 0.5mol/L�� ǡ����ȫ��Ӧ������B�����������Է���������40�����B��þ����A��Na��C��Al��D��Cl��E��O��
ǡ����ȫ��Ӧ������B�����������Է���������40�����B��þ����A��Na��C��Al��D��Cl��E��O��
��2�����������к������Ӽ��ͷǼ��Լ���
��3���Ȼ�þ�����ӻ�������γɹ���Ϊ
 ��
��
��4�������������Ʒ�Ӧ�ķ���ʽΪ2OH-+2Al +2H2O=2AlO2-+3 H2����
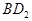 ��˵��B�ǵڢ�A��D�ǵ���A��2gB����������100ml 0.5mol/L��
��˵��B�ǵڢ�A��D�ǵ���A��2gB����������100ml 0.5mol/L�� ǡ����ȫ��Ӧ������B�����������Է���������40�����B��þ����A��Na��C��Al��D��Cl��E��O��
ǡ����ȫ��Ӧ������B�����������Է���������40�����B��þ����A��Na��C��Al��D��Cl��E��O����2�����������к������Ӽ��ͷǼ��Լ���
��3���Ȼ�þ�����ӻ�������γɹ���Ϊ
 ��
����4�������������Ʒ�Ӧ�ķ���ʽΪ2OH-+2Al +2H2O=2AlO2-+3 H2����

��ϰ��ϵ�д�
�����Ŀ